Validity of the Patient Experiences and Satisfaction with Medications (PESaM) Questionnaire
- PMID: 30367435
- PMCID: PMC6335379
- DOI: 10.1007/s40271-018-0340-6
Validity of the Patient Experiences and Satisfaction with Medications (PESaM) Questionnaire
Abstract
Background: This study assessed the validity and reliability of the generic module of the recently developed Patient Experiences and Satisfaction with Medications (PESaM) questionnaire in a sample of patients in the Netherlands.
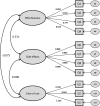
Methods: The generic module of the PESaM questionnaire consists of 18 items related to the domains effectiveness, side effects and ease of use of medications. It assesses patients' experiences regarding the impact of the medication on daily life, health and satisfaction. In 2017, the PESaM questionnaire was sent out to idiopathic pulmonary fibrosis patients using pirfenidone or nintedanib, atypical haemolytic uraemic syndrome patients receiving eculizumab and patients using tacrolimus after kidney transplantation. Mean scores for each domain were calculated applying a scoring algorithm. Construct validity and reliability were assessed using recommended methods.
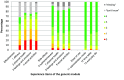
Results: 188 participants completed the generic module, of whom 48% used pirfenidone, 36% nintedanib, 11% tacrolimus and 5% eculizumab. The generic module has good structural properties. Internal consistency values of the domains were satisfactory (i.e. Cronbach's coefficient alpha above 0.7). Confirmatory factor analysis provided further evidence for construct validity, with good convergent and discriminant validity. The PESaM questionnaire also showed different scores for patients using different medications, in line with expectations, and was therefore able to differentiate between patient groups. Test-retest reliability of the items and domains were rated as moderate to fair (i.e. intraclass coefficients ranged between 0.18 and 0.76).
Conclusions: The PESaM questionnaire is a unique patient-reported outcome measure evaluating patient experiences and satisfaction with medications. It has been developed in conjunction with patients, ensuring coverage of domains and issues relevant from the patient's perspective. This study has shown promising validity of the generic module of the PESaM questionnaire. Further research is recommended to assess reliability in greater detail as well as the responsiveness of the measure.
Trial registration: The study is registered in The Netherlands National Trial Register (Trial Code 5860).
Conflict of interest statement
Merel Kimman, Marlies Wijsenbeek, Sander van Kuijk, Kioa Wijnsma, Nicole van de Kar, Marjolein Storm, Xana van Jaarsveld, Carmen Dirksen, Paul Bresser, Ilona Coenen, Nynke Dijkstra, Brigitte van Dooren, Miranda Geelhoed, Marielle Gelens, Kirsten de Haas, René Jonkers, Bart Koopman, Henk Kramer, Mirjam van Manen, Jelle Miedema, Karen Moor, Rémy Mostard, Esther Nossent, Marieke Overbeek, Ellen Peeters, Rein van Rijswijk, Helen Ryan, Nelleke Tak, Anneke van Veen, Lucyl Verhoeven, Michiel de Vries, Monique Wapenaar, Ingrid Wegman and Jack Wetzels have no conflicts of interest, including non-financial, that are directly relevant to the contents of this manuscript.
Figures
References
-
- Hirji I, Gupta S, Goren A, Chirovsky DR, Moadel AB, Olavarria E, et al. Chronic myeloid leukemia (CML): association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient’s perspective. Health Qual Life Outcomes. 2013;11:167. doi: 10.1186/1477-7525-11-167. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

