Loss of amphiregulin reduces myoepithelial cell coverage of mammary ducts and alters breast tumor growth
- PMID: 30367629
- PMCID: PMC6203982
- DOI: 10.1186/s13058-018-1057-0
Loss of amphiregulin reduces myoepithelial cell coverage of mammary ducts and alters breast tumor growth
Abstract
Background: Amphiregulin (AREG), a ligand of the epidermal growth factor receptor, is not only essential for proper mammary ductal development, but also associated with breast cancer proliferation and growth. In the absence of AREG, mammary ductal growth is stunted and fails to expand. Furthermore, suppression of AREG expression in estrogen receptor-positive breast tumor cells inhibits in-vitro and in-vivo growth.
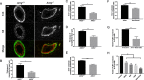
Methods: We crossed AREG-null (AREG-/-) mice with the murine luminal B breast cancer model, MMTV-PyMT (PyMT), to generate spontaneous breast tumors that lack AREG (AREG-/- PyMT). We evaluated tumor growth, cytokeratin-8 (K8)-positive luminal cells, cytokeratin-14 (K14)-positive myoepithelial cells, and expression of AREG, Ki67, and PyMT. Primary myoepithelial cells from nontumor-bearing AREG+/+ mice underwent fluorescence-activated cell sorting and were adapted to culture for in-vitro coculture studies with AT-3 cells, a cell line derived from C57Bl/6 PyMT mammary tumors.
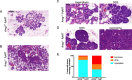
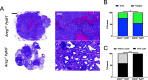
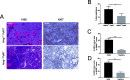
Results: Intriguingly, PyMT-induced lesions progress more rapidly in AREG-/- mice than in AREG+/+ mice. Quantification of K8+ luminal and K14+ myoepithelial cells in non-PyMT AREG-/- mammary glands showed fewer K14+ cells and a thinner myoepithelial layer. Study of AT-3 cells indicated that coculture with myoepithelial cells or exposure to AREG, epidermal growth factor, or basic fibroblast growth factor can suppress PyMT expression. Late-stage AREG-/- PyMT tumors are significantly less solid in structure, with more areas of papillary and cystic growth. Papillary areas appear to be both less proliferative and less necrotic. In The Cancer Genome Atlas database, luminal-B invasive papillary carcinomas have lower AREG expression than luminal B invasive ductal carcinomas.
Conclusions: Our study has revealed a previously unknown role of AREG in myoepithelial cell development and PyMT expression. AREG expression is essential for proper myoepithelial coverage of mammary ducts. Both AREG and myoepithelial cells can suppress PyMT expression. We find that lower AREG expression is associated with invasive papillary breast cancer in both the MMTV-PyMT model and human breast cancer.
Keywords: Amphiregulin; Breast cancer; MMTV-PyMT; Mammary ductal development.
Conflict of interest statement
Ethics approval and consent to participate
The animal studies were completed in line with our animal protocol with approval by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Transformation of enriched mammary cell populations with polyomavirus middle T antigen influences tumor subtype and metastatic potential.Breast Cancer Res. 2015 Oct 1;17(1):132. doi: 10.1186/s13058-015-0641-9. Breast Cancer Res. 2015. PMID: 26429062 Free PMC article.
-
Preneoplastic mammary tumor markers: Cripto and Amphiregulin are overexpressed in hyperplastic stages of tumor progression in transgenic mice.Int J Cancer. 1999 May 17;81(4):588-91. doi: 10.1002/(sici)1097-0215(19990517)81:4<588::aid-ijc14>3.0.co;2-i. Int J Cancer. 1999. PMID: 10225449
-
Frequent overexpression of AMAP1, an Arf6 effector in cell invasion, is characteristic of the MMTV-PyMT rather than the MMTV-Neu human breast cancer model.Cell Commun Signal. 2018 Jan 5;16(1):1. doi: 10.1186/s12964-017-0212-z. Cell Commun Signal. 2018. PMID: 29329590 Free PMC article.
-
Insights from transgenic mouse models of PyMT-induced breast cancer: recapitulating human breast cancer progression in vivo.Oncogene. 2021 Jan;40(3):475-491. doi: 10.1038/s41388-020-01560-0. Epub 2020 Nov 24. Oncogene. 2021. PMID: 33235291 Free PMC article. Review.
-
The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma.Oncogene. 2000 Feb 21;19(8):1020-7. doi: 10.1038/sj.onc.1203280. Oncogene. 2000. PMID: 10713685 Review.
Cited by
-
TGF-β1 stimulates epithelial-mesenchymal transition and cancer-associated myoepithelial cell during the progression from in situ to invasive breast cancer.Cancer Cell Int. 2019 Dec 19;19:343. doi: 10.1186/s12935-019-1068-7. eCollection 2019. Cancer Cell Int. 2019. PMID: 31889895 Free PMC article.
-
Live-Cell Sender-Receiver Co-cultures for Quantitative Measurement of Paracrine Signaling Dynamics, Gene Expression, and Drug Response.Methods Mol Biol. 2023;2634:285-314. doi: 10.1007/978-1-0716-3008-2_13. Methods Mol Biol. 2023. PMID: 37074584
References
-
- Sainsbury JR, et al. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987;1(8547):1398–1402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

