Selective activation and proliferation of a quiescent stem cell population in the neuroepithelial body microenvironment
- PMID: 30367659
- PMCID: PMC6203996
- DOI: 10.1186/s12931-018-0915-8
Selective activation and proliferation of a quiescent stem cell population in the neuroepithelial body microenvironment
Abstract
Background: The microenvironment (ME) of neuroepithelial bodies (NEBs) harbors densely innervated groups of pulmonary neuroendocrine cells that are covered by Clara-like cells (CLCs) and is believed to be important during development and for adult airway epithelial repair after severe injury. Yet, little is known about its potential stem cell characteristics in healthy postnatal lungs.
Methods: Transient mild lung inflammation was induced in mice via a single low-dose intratracheal instillation of lipopolysaccharide (LPS). Bronchoalveolar lavage fluid (BALF), collected 16 h after LPS instillation, was used to challenge the NEB ME in ex vivo lung slices of control mice. Proliferating cells in the NEB ME were identified and quantified following simultaneous LPS instillation and BrdU injection.
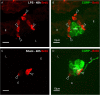
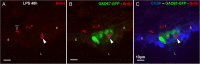
Results: The applied LPS protocol induced very mild and transient lung injury. Challenge of lung slices with BALF of LPS-treated mice resulted in selective Ca2+-mediated activation of CLCs in the NEB ME of control mice. Forty-eight hours after LPS challenge, a remarkably selective and significant increase in the number of divided (BrdU-labeled) cells surrounding NEBs was observed in lung sections of LPS-challenged mice. Proliferating cells were identified as CLCs.
Conclusions: A highly reproducible and minimally invasive lung inflammation model was validated for inducing selective activation of a quiescent stem cell population in the NEB ME. The model creates new opportunities for unraveling the cellular mechanisms/pathways regulating silencing, activation, proliferation and differentiation of this unique postnatal airway epithelial stem cell population.
Keywords: Airway epithelium; Clara-like cells; Lipopolysaccharide; Neuroepithelial body microenvironment; Proliferation; Pulmonary neuroendocrine cells; Stem cell niche.
Conflict of interest statement
Ethics approval
National and international principles of laboratory animal care were followed, and experiments were approved by the local animal ethics committee of the University of Antwerp (ECD 2014–66 and 2017–49).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures









References
-
- Thurlbeck WM. Postnatal growth and development of the lung. Am Rev Respir Dis. 1975;111:803–844. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

