Neuronal extracellular microRNAs miR-124 and miR-9 mediate cell-cell communication between neurons and microglia
- PMID: 30367726
- PMCID: PMC6587827
- DOI: 10.1002/jnr.24344
Neuronal extracellular microRNAs miR-124 and miR-9 mediate cell-cell communication between neurons and microglia
Abstract
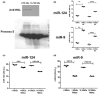
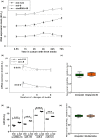
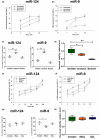
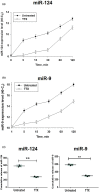
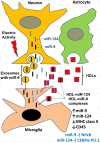
In contrast to peripheral macrophages, microglia in the central nervous system (CNS) exhibit a specific deactivated phenotype; however, it is not clear how this phenotype is maintained. Two alternative hypotheses were postulated recently: (a) microglia differ from peripheral macrophages being derived from the yolk sac (YS), whereas peripheral macrophages originate from bone marrow (BM); (b) microglia acquire a specific phenotype under the influence of the CNS microenvironment. We have previously shown that microglia express miR-124, which was also induced in BM-derived macrophages co-cultured with a neurons. We here investigated the possibility of horizontal transfer of the neuron-specific microRNAs miR-124 and miR-9 from primary neurons to microglia/macrophages. We found that after incubation with neuronal conditioned media (NCM), macrophages downregulated activation markers MHC class II and CD45. Neither cultured adult microglia nor YS- and BM-derived macrophages demonstrated intrinsic levels of miR-124 expression. However, after incubation with NCM, miR-124 was induced in both YS- and BM-derived macrophages. Biochemical analysis demonstrated that the NCM contained miR-124 and miR-9 in complex with small proteins, large high-density lipoproteins (HDLs), and exosomes. MiR-124 and miR-9 were promptly released from neurons, and this process was inhibited by tetrodotoxin, indicating an important role of neuronal electric activity in secretion of these microRNAs. Incubation of macrophages with exogenous miR-124 resulted in efficient translocation of miR-124 into the cytoplasm. This study demonstrates an important role of neuronal miRNAs in communication of neurons with microglia, which favors the hypothesis that microglia acquire a specific phenotype under the influence of the CNS microenvironment.
Keywords: HDL; extracellular miRNA; macrophages; miR-124; microglia.
© 2018 The Authors. Journal of Neuroscience Research Published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury.J Neuroinflammation. 2020 Mar 19;17(1):89. doi: 10.1186/s12974-020-01761-0. J Neuroinflammation. 2020. PMID: 32192523 Free PMC article.
-
Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes.J Nanobiotechnology. 2020 Jul 25;18(1):105. doi: 10.1186/s12951-020-00665-8. J Nanobiotechnology. 2020. PMID: 32711535 Free PMC article.
-
Secretome from SH-SY5Y APPSwe cells trigger time-dependent CHME3 microglia activation phenotypes, ultimately leading to miR-21 exosome shuttling.Biochimie. 2018 Dec;155:67-82. doi: 10.1016/j.biochi.2018.05.015. Epub 2018 May 29. Biochimie. 2018. PMID: 29857185
-
MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS.Glia. 2013 Jan;61(1):91-103. doi: 10.1002/glia.22363. Epub 2012 May 31. Glia. 2013. PMID: 22653784 Free PMC article. Review.
-
Vesicular Transport of Encapsulated microRNA between Glial and Neuronal Cells.Int J Mol Sci. 2020 Jul 18;21(14):5078. doi: 10.3390/ijms21145078. Int J Mol Sci. 2020. PMID: 32708414 Free PMC article. Review.
Cited by
-
Circular RNA HIPK2 Promotes A1 Astrocyte Activation after Spinal Cord Injury through Autophagy and Endoplasmic Reticulum Stress by Modulating miR-124-3p-Mediated Smad2 Repression.ACS Omega. 2023 Dec 26;9(1):781-797. doi: 10.1021/acsomega.3c06679. eCollection 2024 Jan 9. ACS Omega. 2023. PMID: 38222662 Free PMC article.
-
The Role of Neuronal Factors in the Epigenetic Reprogramming of Microglia in the Normal and Diseased Central Nervous System.Front Cell Neurosci. 2019 Oct 11;13:453. doi: 10.3389/fncel.2019.00453. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31680868 Free PMC article. Review.
-
Chronic Regulation of miR-124-3p in the Perilesional Cortex after Experimental and Human TBI.Int J Mol Sci. 2020 Mar 31;21(7):2418. doi: 10.3390/ijms21072418. Int J Mol Sci. 2020. PMID: 32244461 Free PMC article.
-
Hydrogen-rich saline alleviates cardiomyocyte apoptosis by reducing expression of calpain1 via miR-124-3p.ESC Heart Fail. 2023 Oct;10(5):3077-3090. doi: 10.1002/ehf2.14492. Epub 2023 Aug 21. ESC Heart Fail. 2023. PMID: 37602925 Free PMC article.
-
Neuronal and Glial Communication via Non-Coding RNAs: Messages in Extracellular Vesicles.Int J Mol Sci. 2022 Dec 28;24(1):470. doi: 10.3390/ijms24010470. Int J Mol Sci. 2022. PMID: 36613914 Free PMC article. Review.
References
-
- Barteneva, N. S. , Baiken, Y. , Fasler‐Kan, E. , Alibek, K. , Wang, S. , Maltsev, N. , … Vorobjev, I. A. (2017). Extracellular vesicles in gastrointestinal cancer in conjunction with microbiota: On the border of Kingdoms. Biochimica Et Biophysica Acta ‐ Reviews on Cancer, 1868(2), 372–393. 10.1016/j.bbcan.2017.06.005 - DOI - PubMed
-
- Bazzoni, F. , Rossato, M. , Fabbri, M. , Gaudiosi, D. , Mirolo, M. , Mori, L. , … Locati, M. (2009). Induction and regulatory function of miR‐9 in human monocytes and neutrophils exposed to proinflammatory signals. Proceedings of the National Academy of Sciences, 106(13), 5282–5287. 10.1073/pnas.0810909106 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

