Biocompatibility of common implantable sensor materials in a tumor xenograft model
- PMID: 30367816
- PMCID: PMC6767110
- DOI: 10.1002/jbm.b.34254
Biocompatibility of common implantable sensor materials in a tumor xenograft model
Abstract
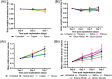
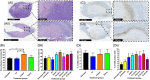
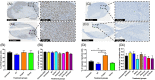
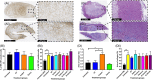
Real-time monitoring of tumor microenvironment parameters using an implanted biosensor could provide valuable information on the dynamic nature of a tumor's biology and its response to treatment. However, following implantation biosensors may lose functionality due to biofouling caused by the foreign body response (FBR). This study developed a novel tumor xenograft model to evaluate the potential of six biomaterials (silicon dioxide, silicon nitride, Parylene-C, Nafion, biocompatible EPOTEK epoxy resin, and platinum) to trigger a FBR when implanted into a solid tumor. Biomaterials were chosen based on their use in the construction of a novel biosensor, designed to measure spatial and temporal changes in intra-tumoral O2 , and pH. None of the biomaterials had any detrimental effect on tumor growth or body weight of the murine host. Immunohistochemistry showed no significant changes in tumor necrosis, hypoxic cell number, proliferation, apoptosis, immune cell infiltration, or collagen deposition. The absence of biofouling supports the use of these materials in biosensors; future investigations in preclinical cancer models are required, with a view to eventual applications in humans. To our knowledge this is the first documented investigation of the effects of modern biomaterials, used in the production of implantable sensors, on tumor tissue after implantation. © 2018 The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials published by Wiley Periodicals, Inc. J Biomed Mater Res Part B, 2018. © 2018 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater 107B: 1620-1633, 2019.
Keywords: biocompatibility; foreign body response; implantable biosensor; innate immune response; tumor microenvironment; tumor xenograft model.
© 2018 The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials published by Wiley Periodicals, Inc.
Conflict of interest statement
There are no conflict of interest.
Figures


References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):359–386. - PubMed
-
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 2004;14(3):198–206. - PubMed
-
- Beyer GP, Mann GG, Pursley JA, Espenhahn ET, Fraisse C, Godfrey DJ, Oldham M, Carrea TB, Bolick N, Scarantino CW. An implantable MOSFET dosimeter for the measurement of radiation dose in tissue during cancer therapy. IEEE Sensors J 2008;8(1):38–51.
-
- Marland JRK, Blair EO, Flynn BW, González‐Fernández E, Huang L, Kunkler IH, Smith S, Staderini M, Tsiamis A, Ward C and Murray A. Implantable microsystems for personalised anticancer therapy In: Mitra S and Cumming DRS, editors. CMOS Circuits for Biological Sensing and Processing. UK: Springer International Publishing; 2018, pp. 259–286.
-
- Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, Richter C, Zips D, Bortfeld T. Radiation oncology in the era of precision medicine. Nat Rev Cancer 2016;16:234–249. - PubMed

