Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury
- PMID: 30367898
- PMCID: PMC6704477
- DOI: 10.1016/j.jhep.2018.10.015
Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury
Erratum in
-
Corrigendum to: "Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury" [J Hepatol (2019) 458-469].J Hepatol. 2020 Sep;73(3):744. doi: 10.1016/j.jhep.2020.05.034. Epub 2020 Jun 27. J Hepatol. 2020. PMID: 32605877 No abstract available.
Abstract
Background & aims: Endothelial dysfunction plays an essential role in liver injury, yet the phenotypic regulation of liver sinusoidal endothelial cells (LSECs) remains unknown. Autophagy is an endogenous protective system whose loss could undermine LSEC integrity and phenotype. The aim of our study was to investigate the role of autophagy in the regulation of endothelial dysfunction and the impact of its manipulation during liver injury.
Methods: We analyzed primary isolated LSECs from Atg7control and Atg7endo mice as well as rats after CCl4 induced liver injury. Liver tissue and primary isolated stellate cells were used to analyze liver fibrosis. Autophagy flux, microvascular function, nitric oxide bioavailability, cellular superoxide content and the antioxidant response were evaluated in endothelial cells.
Results: Autophagy maintains LSEC homeostasis and is rapidly upregulated during capillarization in vitro and in vivo. Pharmacological and genetic downregulation of endothelial autophagy increases oxidative stress in vitro. During liver injury in vivo, the selective loss of endothelial autophagy leads to cellular dysfunction and reduced intrahepatic nitric oxide. The loss of autophagy also impairs LSECs ability to handle oxidative stress and aggravates fibrosis.
Conclusions: Autophagy contributes to maintaining endothelial phenotype and protecting LSECs from oxidative stress during early phases of liver disease. Selectively potentiating autophagy in LSECs during early stages of liver disease may be an attractive approach to modify the disease course and prevent fibrosis progression.
Lay summary: Liver endothelial cells are the first liver cell type affected after any kind of liver injury. The loss of their unique phenotype during injury amplifies liver damage by orchestrating the response of the liver microenvironment. Autophagy is a mechanism involved in the regulation of this initial response and its manipulation can modify the progression of liver damage.
Keywords: Atg7; Autophagy; Endothelial cell; Endothelial dysfunction; LSEC; Liver fibrosis; Nitric oxide; Nrf2; Oxidative stress; eNOS.
Copyright © 2018 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


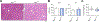
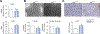


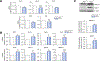
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

