Integrative approach identifies corticosteroid response variant in diverse populations with asthma
- PMID: 30367910
- PMCID: PMC6482107
- DOI: 10.1016/j.jaci.2018.09.034
Integrative approach identifies corticosteroid response variant in diverse populations with asthma
Abstract
Background: Although inhaled corticosteroid (ICS) medication is considered the cornerstone treatment for patients with persistent asthma, few ICS pharmacogenomic studies have involved nonwhite populations.
Objective: We sought to identify genetic predictors of ICS response in multiple population groups with asthma.
Methods: The discovery group comprised African American participants from the Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-Ethnicity (SAPPHIRE) who underwent 6 weeks of monitored ICS therapy (n = 244). A genome-wide scan was performed to identify single nucleotide polymorphism (SNP) variants jointly associated (ie, the combined effect of the SNP and SNP × ICS treatment interaction) with changes in asthma control. Top associations were validated by assessing the joint association with asthma exacerbations in 3 additional groups: African Americans (n = 803 and n = 563) and Latinos (n = 1461). RNA sequencing data from 408 asthmatic patients and 405 control subjects were used to examine whether genotype was associated with gene expression.
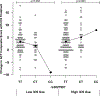
Results: One variant, rs3827907, was significantly associated with ICS-mediated changes in asthma control in the discovery set (P = 7.79 × 10-8) and was jointly associated with asthma exacerbations in 3 validation cohorts (P = .023, P = .029, and P = .041). RNA sequencing analysis found the rs3827907 C-allele to be associated with lower RNASE2 expression (P = 6.10 × 10-4). RNASE2 encodes eosinophil-derived neurotoxin, and the rs3827907 C-allele appeared to particularly influence ICS treatment response in the presence of eosinophilic inflammation (ie, high pretreatment eosinophil-derived neurotoxin levels or blood eosinophil counts).
Conclusion: We identified a variant, rs3827907, that appears to influence response to ICS treatment in multiple population groups and likely mediates its effect through eosinophils.
Keywords: EDDM3B; Pharmacogenetics; RNASE2; eosinophil-derived neurotoxin; eosinophils; transcriptome.
Copyright © 2018 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 2012:1–8. - PubMed
-
- Sin DD, Man J, Sharpe H, Gan WQ, Man SF. Pharmacological management to reduce exacerbations in adults with asthma: a systematic review and meta-analysis. JAMA 2004; 292:367–76. - PubMed
-
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343:332–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 HL135403/HL/NHLBI NIH HHS/United States
- R01 DK113003/DK/NIDDK NIH HHS/United States
- R01 AI079139/AI/NIAID NIH HHS/United States
- R01 HL141845/HL/NHLBI NIH HHS/United States
- R01 HL141992/HL/NHLBI NIH HHS/United States
- T32 GM007546/GM/NIGMS NIH HHS/United States
- U19 AI077439/AI/NIAID NIH HHS/United States
- R01 HL079055/HL/NHLBI NIH HHS/United States
- R21 ES024844/ES/NIEHS NIH HHS/United States
- K99 HL135403/HL/NHLBI NIH HHS/United States
- R01 HL118267/HL/NHLBI NIH HHS/United States
- R01 MD010443/MD/NIMHD NIH HHS/United States
- R01 ES015794/ES/NIEHS NIH HHS/United States
- R01 AI061774/AI/NIAID NIH HHS/United States
- R01 HL117004/HL/NHLBI NIH HHS/United States
- P60 MD006902/MD/NIMHD NIH HHS/United States
- R01 DK064695/DK/NIDDK NIH HHS/United States

