Association of Pretransplant Renal Function With Liver Graft and Patient Survival After Liver Transplantation in Patients With Nonalcoholic Steatohepatitis
- PMID: 30369023
- PMCID: PMC6709989
- DOI: 10.1002/lt.25367
Association of Pretransplant Renal Function With Liver Graft and Patient Survival After Liver Transplantation in Patients With Nonalcoholic Steatohepatitis
Abstract
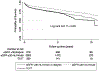
Nonalcoholic steatohepatitis (NASH) is one of the top 3 indications for liver transplantation (LT) in Western countries. It is unknown whether renal dysfunction at the time of LT has any effect on post-LT outcomes in recipients with NASH. From the United Network for Organ Sharing-Standard Transplant Analysis and Research data set, we identified 4088 NASH recipients who received deceased donor LT. We divided our recipients a priori into 3 categories: group 1 with estimated glomerular filtration rate (eGFR) <30 mL/minute/1.73 m2 at the time of LT and/or received dialysis within 2 weeks preceding LT (n = 937); group 2 with recipients who had eGFR ≥30 mL/minute/1.73 m2 and who did not receive renal replacement therapy prior to LT (n = 2812); and group 3 with recipients who underwent simultaneous liver-kidney transplantation (n = 339). We examined the association of pretransplant renal dysfunction with death with a functioning graft, all-cause mortality, and graft loss using competing risk regression and Cox proportional hazards models. The mean ± standard deviation age of the cohort at baseline was 58 ± 8 years, 55% were male, 80% were Caucasian, and average exception Model for End-Stage Liver Disease score was 24 ± 9. The median follow-up period was 5 years (median, 1816 days; interquartile range, 1090-2723 days). Compared with group 1 recipients, group 2 recipients had 19% reduced trend for risk for death with a functioning graft (subhazard ratio [SHR], 0.81; 95% confidence interval [CI], 0.64-1.02) and similar risk for graft loss (SHR, 1.25; 95% CI, 0.59-2.62), whereas group 3 recipients had similar risk for death with a functioning graft (SHR, 1.23; 95% CI, 0.96-1.57) and graft loss (SHR, 0.18; 95% CI, 0.02-1.37) using an adjusted competing risk regression model. In conclusion, recipients with preserved renal function before LT showed a trend toward lower risk of death with a functioning graft compared with SLKT recipients and those with pretransplant severe renal dysfunction in patients with NASH.
Copyright © 2018 by the American Association for the Study of Liver Diseases.
Figures


Comment in
-
Predicting Outcomes of Liver Transplantation in Patients With Nonalcoholic Steatohepatitis: Pretransplant Renal Function Is Key.Liver Transpl. 2019 Mar;25(3):362-364. doi: 10.1002/lt.25413. Liver Transpl. 2019. PMID: 30657244 No abstract available.
References
-
- Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003;349:931–940. - PubMed
-
- Weber ML, Ibrahim HN, Lake JR. Renal dysfunction in liver transplant recipients: evaluation of the critical issues. Liver Transpl 2012;18:1290–1301. - PubMed
-
- Parajuli S, Foley D, Djamali A, Mandelbrot D. Renal function and transplantation in liver disease. Transplantation 2015;99:1756–1764. - PubMed
-
- Calmus Y, Conti F, Cluzel P, Hill G, Antoine C, Scatton O, et al. Prospective assessment of renal histopathological lesions in patients with end-stage liver disease: effects on long-term renal function after liver transplantation. J Hepatol 2012;57:572–576. - PubMed
-
- Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant 2006;6:2651–2659. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

