Immunomodulation by Helminths: Intracellular Pathways and Extracellular Vesicles
- PMID: 30369927
- PMCID: PMC6194161
- DOI: 10.3389/fimmu.2018.02349
Immunomodulation by Helminths: Intracellular Pathways and Extracellular Vesicles
Abstract
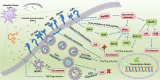
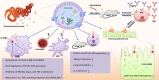
Helminth parasites are masters at manipulating host immune responses, using an array of sophisticated mechanisms. One of the major mechanisms enabling helminths to establish chronic infections is the targeting of pattern recognition receptors (PRRs) including toll-like receptors, C-type lectin receptors, and the inflammasome. Given the critical role of these receptors and their intracellular pathways in regulating innate inflammatory responses, and also directing adaptive immunity toward Th1 and Th2 responses, recognition of the pathways triggered and/or modulated by helminths and their products will provide detailed insights about how helminths are able to establish an immunoregulatory environment. However, helminths also target PRRs-independent mechanisms (and most likely other yet unknown mechanisms and pathways) underpinning the battery of different molecules helminths produce. Herein, the current knowledge on intracellular pathways in antigen presenting cells activated by helminth-derived biomolecules is reviewed. Furthermore, we discuss the importance of helminth-derived vesicles as a less-appreciated components released during infection, their role in activating these host intracellular pathways, and their implication in the development of new therapeutic approaches for inflammatory diseases and the possibility of designing a new generation of vaccines.
Keywords: extracellular vesicle; helminths; immunosuppression; intracellular pathways; pattern recognition receptors.
Figures

References
-
- Jackson JA, Friberg IM, Little S, Bradley JE. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology (2009) 126:18–27. 10.1111/j.1365-2567.2008.03010.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

