Beclin 1 Phosphorylation - at the Center of Autophagy Regulation
- PMID: 30370269
- PMCID: PMC6194997
- DOI: 10.3389/fcell.2018.00137
Beclin 1 Phosphorylation - at the Center of Autophagy Regulation
Abstract
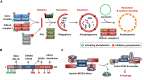
Autophagy is a tightly regulated catabolic process wherein cells under stress sequester cytosolic constituents like damaged proteins and organelles in double-membrane vesicles called autophagosomes. The autophagosomes degrade their cargo by lysosomal proteolysis generating raw materials for the biosynthesis of vital macromolecules. One of the initial steps in the assembly of autophagosomes from pre-autophagic structures is the recruitment and activation of the class III phosphatidylinositol 3-kinase complex consisting of Beclin 1 (BECN1), VPS34, VPS15, and ATG14 proteins. Several pieces of evidence indicate that the phosphorylation and ubiquitination of BECN1 at an array of residues fine-tune the responses to diverse autophagy modulating stimuli and helps in maintaining the balance between pro-survival autophagy and pro-apoptotic responses. In this mini-review, we will discuss the importance of distinct BECN1 phosphorylation events, the diverse signaling pathways and kinases involved and their role in the regulation of autophagy.
Keywords: ATG; BECN1; PI3K; autophagy; beclin; kinase; phosphorylation.
Figures
Similar articles
-
Nrbf2 protein suppresses autophagy by modulating Atg14L protein-containing Beclin 1-Vps34 complex architecture and reducing intracellular phosphatidylinositol-3 phosphate levels.J Biol Chem. 2014 Sep 19;289(38):26021-26037. doi: 10.1074/jbc.M114.561134. Epub 2014 Aug 1. J Biol Chem. 2014. PMID: 25086043 Free PMC article.
-
GLIPR2 is a negative regulator of autophagy and the BECN1-ATG14-containing phosphatidylinositol 3-kinase complex.Autophagy. 2021 Oct;17(10):2891-2904. doi: 10.1080/15548627.2020.1847798. Epub 2020 Nov 23. Autophagy. 2021. PMID: 33222586 Free PMC article.
-
NRBF2 regulates macroautophagy as a component of Vps34 Complex I.Biochem J. 2014 Jul 15;461(2):315-22. doi: 10.1042/BJ20140515. Biochem J. 2014. PMID: 24785657 Free PMC article.
-
Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation.Cell Death Differ. 2019 Mar;26(4):617-629. doi: 10.1038/s41418-018-0254-9. Epub 2018 Dec 13. Cell Death Differ. 2019. PMID: 30546075 Free PMC article. Review.
-
Regulation of the Tumor-Suppressor BECLIN 1 by Distinct Ubiquitination Cascades.Int J Mol Sci. 2017 Nov 27;18(12):2541. doi: 10.3390/ijms18122541. Int J Mol Sci. 2017. PMID: 29186924 Free PMC article. Review.
Cited by
-
Quercetin Might Promote Autophagy in a Middle Cerebral Artery Occlusion-Mediated Ischemia Model: Comments on Fawad-Ali Shah et al.Neurochem Res. 2019 Feb;44(2):297-300. doi: 10.1007/s11064-018-2692-7. Epub 2018 Dec 4. Neurochem Res. 2019. PMID: 30515707 No abstract available.
-
Regulation of Autophagy by the Glycogen Synthase Kinase-3 (GSK-3) Signaling Pathway.Int J Mol Sci. 2022 Feb 1;23(3):1709. doi: 10.3390/ijms23031709. Int J Mol Sci. 2022. PMID: 35163631 Free PMC article. Review.
-
Cell Death Mechanisms in Esophageal Squamous Cell Carcinoma Induced by Vesicular Stomatitis Virus Matrix Protein.Osong Public Health Res Perspect. 2019 Aug;10(4):246-252. doi: 10.24171/j.phrp.2019.10.4.08. Osong Public Health Res Perspect. 2019. PMID: 31497497 Free PMC article.
-
Mechanisms of Prostate Cancer Cells Survival and Their Therapeutic Targeting.Int J Mol Sci. 2023 Feb 2;24(3):2939. doi: 10.3390/ijms24032939. Int J Mol Sci. 2023. PMID: 36769263 Free PMC article. Review.
-
In vitro and in vivo protective potential of quercetin-3-glucuronide against lipopolysaccharide-induced pulmonary injury through dual activation of nuclear factor-erythroid 2 related factor 2 and autophagy.Arch Toxicol. 2024 May;98(5):1415-1436. doi: 10.1007/s00204-024-03691-9. Epub 2024 Mar 4. Arch Toxicol. 2024. PMID: 38436694
References
Publication types
LinkOut - more resources
Full Text Sources

