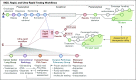
Clinical Utility of Rapid EGFR Genotyping in Advanced Lung Cancer
- PMID: 30370396
- PMCID: PMC6200882
- DOI: 10.1200/PO.17.00299
Clinical Utility of Rapid EGFR Genotyping in Advanced Lung Cancer
Abstract
Purpose: Targeted therapy is the cornerstone of treatment of advanced EGFR-mutant non-small-cell lung cancer (NSCLC). Next-generation sequencing (NGS), the preferred method for genotyping, typically requires several weeks. Here, we assessed workflows designed to rapidly identify patients with actionable EGFR mutations and reduce time to initiation (TTI) of epidermal growth factor receptor (EGFR)-directed therapy.
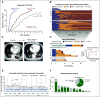
Patients and methods: We performed rapid testing for EGFR L858R mutations and exon 19 deletions on paraffin-embedded or frozen section biopsy specimens from newly diagnosed patients with metastatic NSCLC by using an EGFR-specific assay (rapid test). To determine clinical utility, we assessed concordance with NGS results, turnaround time, and TTI of EGFR therapy, and we evaluated reimbursement data.
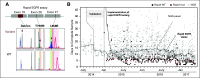
Results: Between January 2015 and September 2017, we performed 243 rapid EGFR tests and identified EGFR mutations in 43 patients (18%). With NGS results as a reference, sensitivity and specificity of the rapid EGFR polymerase chain reaction assay were 98% and 100%, respectively. The median turnaround time for NGS was 14 days, compared with 7 days for rapid testing (P < .001). In the rapid group, 95% of patients received an EGFR inhibitor in the first-line setting. The median TTI of EGFR therapy was significantly shorter in the rapid cohort when compared with 121 historical cases (22 v 37 days; P = .01). Escalation of the initiative into an interdisciplinary ultra-rapid next-day frozen-section workflow for highly symptomatic patients (n = 8) resulted in a reduction in the median (± standard deviation) turnaround time to 1 ± 0.4 days and allowed several patients to initiate therapy within 1 week of biopsy. An extended 9-month clinical evaluation phase confirmed operational sustainability (turnaround times: ultra-rapid, 0.81 ± 0.4 days; rapid, 3 ± 1.5 days), and a 63% reimbursement rate indicated financial sustainability.
Conclusion: Rapid genotyping facilitates earlier initiation of EGFR-directed therapies without compromising NGS workflows.
Conflict of interest statement
Clinical Utility of Rapid
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Ibiayi Dagogo-Jack
Christopher G. Azzoli
Florian Fintelmann
Mari Mino-Kenudson
Anna F. Farago
Justin F. Gainor
Ginger Jiang
Zofia Piotrowska
Rebecca S. Heist
Inga T. Lennes
Jennifer S. Temel
Meghan J. Mooradian
No relationship to disclose
Jessica J. Lin
Subba R. Digumarthy
No relationship to disclose
Julie M. Batten
No relationship to disclose
Hayley Robinson
No relationship to disclose
Vania Nose
No relationship to disclose
Miguel Rivera
Valentina Nardi
Dora Dias-Santagata
No relationship to disclose
Long P. Le
Lecia V. Sequist
Martha Pitman
Jo-Anne O. Shepard
No relationship to disclose
Alice T. Shaw
A. John Iafrate
Jochen K. Lennerz
No relationship to disclose
Figures

References
-
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation–positive non–small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. - PubMed
-
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. - PubMed
-
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

