Reconstructed human keloid models show heterogeneity within keloid scars
- PMID: 30370495
- PMCID: PMC6244653
- DOI: 10.1007/s00403-018-1873-1
Reconstructed human keloid models show heterogeneity within keloid scars
Abstract
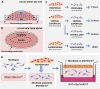
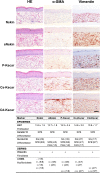
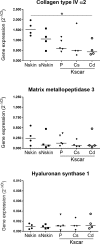
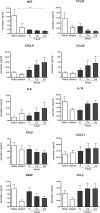
Keloid scars are often described as having an actively growing peripheral margin with a regressing centre. The aim of this study was to examine the possible heterogeneity within keloids and the involvement of different regions within and around keloid scars in the pathogenesis, using an in vitro keloid scar model. In vitro skin models were constructed from keratinocytes and fibroblasts from normal skin and different regions within and around keloid scars: periphery, centre, and (adjacent) surrounding-normal-skin regions. Additionally, fibroblasts were isolated from the superficial-central and deep-central regions of the keloid and combined with central keratinocytes. All keloid regions showed increased contraction compared to normal skin models, particularly in central regions. Myofibroblasts were present in all keloid regions but were more abundant in models containing central-deep keloid fibroblasts. Secretion of anti-fibrotic HGF and extracellular matrix collagen IV gene expression was reduced in the central deep keloid compared to normal skin. No significant differences between peripheral and central regions within keloids were observed for inflammatory cytokine CCL20, CCL27, CXCL8, IL-6 and IL-18 secretion. Parameters for surrounding-normal-skin showed similarities to both non-lesional normal skin and keloids. In conclusion, a simple but elegant method of culturing keloid-derived keratinocytes and fibroblasts in an organotypic 3D scar model was developed, for the dual purpose of studying the underlying pathology and ultimately testing new therapeutics. In this study, these tissue engineered scar models show that the central keloid region shows a more aggressive keloid scar phenotype than the periphery and that the surrounding-normal-skin also shares certain abnormalities characteristic for keloids.
Keywords: Cytokine; Extracellular matrix; In vitro; Keloid; Keloid center; Keloid periphery.
Conflict of interest statement
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval (Research involving Human Participants and/or Animals)
Tissue collection procedures were performed in compliance with the ‘Code for Proper Secondary Use of Human tissue’ as formulated by the Dutch Federation of Medical Scientific Organization (
Informed consent
The discarded skin was collected anonymously if patients had not objected to use of their rest material (opt-out system). Keloid scars (Kscar) were obtained from patients undergoing scar removal via excision. The discarded scar tissue was coded to enable the collection of additional relevant information (e.g. previous treatment, age of scar) and were included only after obtaining oral informed consent.
Figures

Similar articles
-
Characterization of In Vitro Reconstructed Human Normotrophic, Hypertrophic, and Keloid Scar Models.Tissue Eng Part C Methods. 2018 Apr;24(4):242-253. doi: 10.1089/ten.TEC.2017.0464. Epub 2018 Apr 2. Tissue Eng Part C Methods. 2018. PMID: 29490604
-
Hypertrophic and keloid scars fail to progress from the CD34- /α-smooth muscle actin (α-SMA)+ immature scar phenotype and show gradient differences in α-SMA and p16 expression.Br J Dermatol. 2020 Apr;182(4):974-986. doi: 10.1111/bjd.18219. Epub 2019 Sep 4. Br J Dermatol. 2020. PMID: 31206605
-
Deep and superficial keloid fibroblasts contribute differentially to tissue phenotype in a novel in vivo model of keloid scar.Plast Reconstr Surg. 2012 Jun;129(6):1259-1271. doi: 10.1097/PRS.0b013e31824ecaa9. Plast Reconstr Surg. 2012. PMID: 22634643
-
Interplay Between Keratinocytes and Fibroblasts: A Systematic Review Providing a New Angle for Understanding Skin Fibrotic Disorders.Front Immunol. 2020 May 6;11:648. doi: 10.3389/fimmu.2020.00648. eCollection 2020. Front Immunol. 2020. PMID: 32477322 Free PMC article.
-
Keloids and hypertrophic scars: are they two different sides of the same coin?Dermatol Surg. 2008 Mar;34(3):336-46. doi: 10.1111/j.1524-4725.2007.34067.x. Epub 2007 Dec 19. Dermatol Surg. 2008. PMID: 18177398 Review.
Cited by
-
Human In Vitro Skin Models for Wound Healing and Wound Healing Disorders.Biomedicines. 2023 Mar 30;11(4):1056. doi: 10.3390/biomedicines11041056. Biomedicines. 2023. PMID: 37189674 Free PMC article. Review.
-
Construction of a HOXA11-AS-Interact Ed Network in Keloid Fibroblasts Using Integrated Bioinformatic Analysis and in Vitro Validation.Front Genet. 2022 Mar 31;13:844198. doi: 10.3389/fgene.2022.844198. eCollection 2022. Front Genet. 2022. PMID: 35432479 Free PMC article.
-
The Polygenic Map of Keloid Fibroblasts Reveals Fibrosis-Associated Gene Alterations in Inflammation and Immune Responses.Front Immunol. 2022 Jan 10;12:810290. doi: 10.3389/fimmu.2021.810290. eCollection 2021. Front Immunol. 2022. PMID: 35082796 Free PMC article.
-
Towards propagation of epidermal cells for wound repair: glass, as cell culture substrate, enhances proliferation and migration of human keratinocytes.Front Bioeng Biotechnol. 2025 Mar 20;13:1547044. doi: 10.3389/fbioe.2025.1547044. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40182989 Free PMC article.
-
A Reconstructed Human Melanoma-in-Skin Model to Study Immune Modulatory and Angiogenic Mechanisms Facilitating Initial Melanoma Growth and Invasion.Cancers (Basel). 2023 May 20;15(10):2849. doi: 10.3390/cancers15102849. Cancers (Basel). 2023. PMID: 37345186 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

