Coronary arterial vasculature in the pathophysiology of hypertrophic cardiomyopathy
- PMID: 30370501
- PMCID: PMC6481925
- DOI: 10.1007/s00424-018-2224-y
Coronary arterial vasculature in the pathophysiology of hypertrophic cardiomyopathy
Abstract
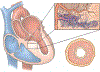
Alterations in the coronary vascular system are likely associated with a mismatch between energy demand and energy supply and critical in triggering the cascade of events that leads to symptomatic hypertrophic cardiomyopathy. Targeting the early events, particularly vascular remodeling, may be a key approach to developing effective treatments. Improvement in our understanding of hypertrophic cardiomyopathy began with the results of early biophysical studies, proceeded to genetic analyses pinpointing the mutational origin, and now pertains to imaging of the metabolic and flow-related consequences of such mutations. Microvascular dysfunction has been an ongoing hot topic in the imaging of genetic cardiomyopathies marked by its histologically significant remodeling and has proven to be a powerful asset in determining prognosis for these patients as well as enlightening scientists on a potential pathophysiological cascade that may begin early during the developmental process. Here, we discuss questions that continue to remain on the mechanistic processes leading to microvascular dysfunction, its correlation to the morphological changes in the vessels, and its contribution to disease progression.
Keywords: Energetics; Hypertrophic cardiomyopathy; Ischemia; Microvascular; Pathogenesis.
Figures
Similar articles
-
Invasive assessment of coronary microvascular dysfunction in hypertrophic cardiomyopathy: the index of microvascular resistance.Cardiovasc Revasc Med. 2015 Oct-Nov;16(7):426-8. doi: 10.1016/j.carrev.2015.06.008. Epub 2015 Jul 11. Cardiovasc Revasc Med. 2015. PMID: 26235975 Review.
-
3.0 T magnetic resonance myocardial perfusion imaging for semi-quantitative evaluation of coronary microvascular dysfunction in hypertrophic cardiomyopathy.Int J Cardiovasc Imaging. 2017 Dec;33(12):1949-1959. doi: 10.1007/s10554-017-1189-9. Epub 2017 Jun 13. Int J Cardiovasc Imaging. 2017. PMID: 28612277
-
Carriers of the hypertrophic cardiomyopathy MYBPC3 mutation are characterized by reduced myocardial efficiency in the absence of hypertrophy and microvascular dysfunction.Eur J Heart Fail. 2011 Dec;13(12):1283-9. doi: 10.1093/eurjhf/hfr135. Epub 2011 Oct 21. Eur J Heart Fail. 2011. PMID: 22021246
-
[Vascular functional alterations in hypertrophic cardiomyopathy].Orv Hetil. 2013 Nov 24;154(47):1851-7. doi: 10.1556/OH.2013.29756. Orv Hetil. 2013. PMID: 24240521 Review. Hungarian.
-
Pathophysiology of chest pain in patients with cardiomyopathies and normal coronary arteries.Circulation. 1982 Apr;65(4):778-89. doi: 10.1161/01.cir.65.4.778. Circulation. 1982. PMID: 7199403
Cited by
-
Myocardial perfusion improvement and mechanism after percutaneous intramyocardial septal radiofrequency ablation in obstructive hypertrophic cardiomyopathy: a study of myocardial contrast echocardiography.Int J Cardiovasc Imaging. 2024 Jul;40(7):1483-1492. doi: 10.1007/s10554-024-03126-7. Epub 2024 May 6. Int J Cardiovasc Imaging. 2024. PMID: 38709352
-
Phenotyping hypertrophic cardiomyopathy using cardiac diffusion magnetic resonance imaging: the relationship between microvascular dysfunction and microstructural changes.Eur Heart J Cardiovasc Imaging. 2022 Feb 22;23(3):352-362. doi: 10.1093/ehjci/jeab210. Eur Heart J Cardiovasc Imaging. 2022. PMID: 34694365 Free PMC article.
-
A Cautionary Tale of Hypertrophic Cardiomyopathy-From "Benign" Left Ventricular Hypertrophy to Stroke, Atrial Fibrillation, and Molecular Genetic Diagnostics: A Case Report and Review of Literature.Int J Mol Sci. 2024 Aug 29;25(17):9385. doi: 10.3390/ijms25179385. Int J Mol Sci. 2024. PMID: 39273332 Free PMC article. Review.
-
Angiotensin II inhibits apoptosis of mouse aortic smooth muscle cells through regulating the circNRG-1/miR-193b-5p/NRG-1 axis.Cell Death Dis. 2019 May 1;10(5):362. doi: 10.1038/s41419-019-1590-5. Cell Death Dis. 2019. PMID: 31043588 Free PMC article.
-
Myocardial contrast echocardiography assessment of perfusion abnormalities in hypertrophic cardiomyopathy.Cardiovasc Ultrasound. 2022 Sep 19;20(1):23. doi: 10.1186/s12947-022-00293-2. Cardiovasc Ultrasound. 2022. PMID: 36117179 Free PMC article. Clinical Trial.
References
-
- Alders DJC, Groeneveld ABJ, de Kanter FJJ, van Beek JHGM (2004) Myocardial O2 consumption in porcine left ventricle is heterogeneously distributed in parallel to heterogeneous O2 delivery. Am J Physiol Heart Circ Physiol 287:H1353–H1361 - PubMed
-
- Alkon J, Friedberg MK, Manlhiot C, Manickaraj AK, Kinnear C, McCrindle BW, Benson LN, Addonizio LJ, Colan SD, Mital S (2012) Genetic variations in hypoxia response genes influence hypertrophic cardiomyopathy phenotype. Pediatr Res 72(6):583–592 - PubMed
-
- Allen DG, Orchard CH (1986) Myocardial contractile function during ischemia and hypoxia. Circulation 60(2):153–168 - PubMed
-
- Alves ML, Dias FAL, Gaffin RD, Simon JN, Montminy EM, Biesiadecki BJ, Hinken AC, Warren CM, Utter MS, Davis RTR, Sakthivel S, Robbins J, Wieczorek DF, Solaro RJ, Wolska BM (2014) Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ Cardiovasc Genet 7(2):132–143 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

