Delivery of shRNA via lentivirus in human pseudoislets provides a model to test dynamic regulation of insulin secretion and gene function in human islets
- PMID: 30370689
- PMCID: PMC6204361
- DOI: 10.14814/phy2.13907
Delivery of shRNA via lentivirus in human pseudoislets provides a model to test dynamic regulation of insulin secretion and gene function in human islets
Abstract
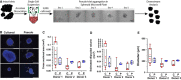
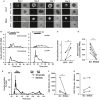
Rodent islets are widely used to study the pathophysiology of beta cells and islet function, however, structural and functional differences exist between human and rodent islets, highlighting the need for human islet studies. Human islets are highly variable, deteriorate during culture, and are difficult to genetically modify, making mechanistic studies difficult to conduct and reproduce. To overcome these limitations, we tested whether pseudoislets, created by dissociation and reaggregation of islet cell suspensions, allow for assessment of dynamic islet function after genetic modulation. Characterization of pseudoislets cultured for 1 week revealed better preservation of first-phase glucose-stimulated insulin secretion (GSIS) compared with cultured-intact islets and insulin secretion profiles similar to fresh islets when challenged by glibenclamide and KCl. qPCR indicated that pseudoislets are similar to the original islets for the expression of markers for cell types, beta cell function, and cellular stress with the exception of reduced proinflammatory cytokine genes (IL1B, CCL2, CXCL8). The expression of extracellular matrix markers (ASPN, COL1A1, COL4A1) was also altered in pseudoislets compared with intact islets. Compared with intact islets transduced by adenovirus, pseudoislets transduced by lentivirus showed uniform transduction and better first-phase GSIS. Lastly, the lentiviral-mediated delivery of short hairpin RNA targeting glucokinase (GCK) achieved significant reduction of GCK expression in pseudoislets as well as marked reduction of both first and second phase GSIS without affecting the insulin secretion in response to KCl. Thus, pseudoislets are a tool that enables efficient genetic modulation of human islet cells while preserving insulin secretion.
Keywords: Beta cell; diabetes; extracellular matrix; glucokinase; inflammation.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures



Similar articles
-
Lentiviral Mediated Gene Silencing in Human Pseudoislet Prepared in Low Attachment Plates.J Vis Exp. 2019 May 14;(147):10.3791/59578. doi: 10.3791/59578. J Vis Exp. 2019. PMID: 31157773 Free PMC article.
-
Glucose, adrenaline and palmitate antagonistically regulate insulin and glucagon secretion in human pseudoislets.Sci Rep. 2019 Jul 16;9(1):10261. doi: 10.1038/s41598-019-46545-6. Sci Rep. 2019. PMID: 31311971 Free PMC article.
-
Signaling in insulin-secreting MIN6 pseudoislets and monolayer cells.J Proteome Res. 2013 Dec 6;12(12):5954-62. doi: 10.1021/pr400864w. Epub 2013 Sep 30. J Proteome Res. 2013. PMID: 24006944
-
Newer perspective on the coupling between glucose-mediated signaling and β-cell functionality.Endocr J. 2020 Jan 28;67(1):1-8. doi: 10.1507/endocrj.EJ19-0335. Epub 2019 Nov 6. Endocr J. 2020. PMID: 31694991 Review.
-
Pancreatic Pseudoislets: An Organoid Archetype for Metabolism Research.Diabetes. 2021 May;70(5):1051-1060. doi: 10.2337/db20-1115. Epub 2021 May 4. Diabetes. 2021. PMID: 33947722 Free PMC article. Review.
Cited by
-
Scaffold-free endocrine tissue engineering: role of islet organization and implications in type 1 diabetes.BMC Endocr Disord. 2025 Apr 21;25(1):107. doi: 10.1186/s12902-025-01919-y. BMC Endocr Disord. 2025. PMID: 40259265 Free PMC article. Review.
-
Functional analysis of islet cells in vitro, in situ, and in vivo.Semin Cell Dev Biol. 2020 Jul;103:14-19. doi: 10.1016/j.semcdb.2020.02.002. Epub 2020 Feb 17. Semin Cell Dev Biol. 2020. PMID: 32081627 Free PMC article. Review.
-
Adipose Triglyceride Lipase Is a Key Lipase for the Mobilization of Lipid Droplets in Human β-Cells and Critical for the Maintenance of Syntaxin 1a Levels in β-Cells.Diabetes. 2020 Jun;69(6):1178-1192. doi: 10.2337/db19-0951. Epub 2020 Apr 20. Diabetes. 2020. PMID: 32312867 Free PMC article.
-
Utilization of commercial collagens for preparing well-differentiated human beta cells for confocal microscopy.Front Endocrinol (Lausanne). 2023 May 25;14:1187216. doi: 10.3389/fendo.2023.1187216. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37305047 Free PMC article.
-
Lentiviral Mediated Gene Silencing in Human Pseudoislet Prepared in Low Attachment Plates.J Vis Exp. 2019 May 14;(147):10.3791/59578. doi: 10.3791/59578. J Vis Exp. 2019. PMID: 31157773 Free PMC article.
References
-
- Arous, C. , and Wehrle‐Haller B.. 2017. Role and impact of the extracellular matrix on integrin‐mediated pancreatic beta‐cell functions. Biol. Cell 109:223–237. - PubMed
-
- Arrojo e Drigo, R. , Ali Y., Diez J., Srinivasan D. K., Berggren P. O., and Boehm B. O.. 2015. New insights into the architecture of the islet of Langerhans: a focused cross‐species assessment. Diabetologia 58:2218–2228. - PubMed
-
- Arzouni, A. A. , Vargas‐Seymour A., Rackham C. L., Dhadda P., Huang G. C., Choudhary P., et al. 2017. Mesenchymal stromal cells improve human islet function through released products and extracellular matrix. Clin. Sci. (Lond.) 131:2835–2845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

