Cellular biology of fracture healing
- PMID: 30370699
- PMCID: PMC6542569
- DOI: 10.1002/jor.24170
Cellular biology of fracture healing
Abstract
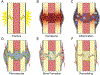
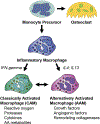
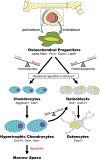
The biology of bone healing is a rapidly developing science. Advances in transgenic and gene-targeted mice have enabled tissue and cell-specific investigations of skeletal regeneration. As an example, only recently has it been recognized that chondrocytes convert to osteoblasts during healing bone, and only several years prior, seminal publications reported definitively that the primary tissues contributing bone forming cells during regeneration were the periosteum and endosteum. While genetically modified animals offer incredible insights into the temporal and spatial importance of various gene products, the complexity and rapidity of healing-coupled with the heterogeneity of animal models-renders studies of regenerative biology challenging. Herein, cells that play a key role in bone healing will be reviewed and extracellular mediators regulating their behavior discussed. We will focus on recent studies that explore novel roles of inflammation in bone healing, and the origins and fates of various cells in the fracture environment. © 2018 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res.
Keywords: bone regeneration; bone repair; fracture healing.
© 2018 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures
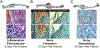

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

