Carotid bodies contribute to sympathoexcitation induced by acute salt overload
- PMID: 30370945
- PMCID: PMC6317332
- DOI: 10.1113/EP087110
Carotid bodies contribute to sympathoexcitation induced by acute salt overload
Abstract
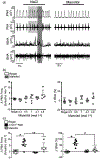
New findings: What is the central question of this study? Does carotid body input contribute to the hyperosmotic responses? What is the main finding and its importance? The response to NaCl overload is sympathorespiratory excitation. Eliminating the carotid body input reduced sympathoexcitation but did not affect the increase in phrenic burst frequency, whereas eliminating the hypothalamus prevented the tachypnoea and sympathoexcitation. We conclude that the carotid body inputs are essential for the full expression of the sympathetic activity during acute NaCl overload, whereas the tachypnoea depends on hypothalamic mechanisms.
Abstract: Acute salt excess activates central osmoreceptors, which trigger an increase in sympathetic and respiratory activity. The carotid bodies also respond to hyperosmolality of the extracellular compartment, but their contribution to the sympathoexcitatory and ventilatory responses to NaCl overload remains unknown. To evaluate their contribution to acute NaCl overload, we recorded thoracic sympathetic (tSNA), phrenic (PNA) and carotid sinus nerve activities in decorticate in situ preparations of male Holtzman rats (60-100 g) while delivering intra-arterial infusions of hyperosmotic NaCl (0.17, 0.3, 0.7, 1.5 and 2.0 mol l-1 ; 200 μl infusion over 25-30 s, with a 10 min time interval between solutions) or mannitol (0.3, 0.5, 1.0, 2.7 and 3.8 mol l-1 ) progressively. The cumulative infusions of hyperosmotic NaCl increased the perfusate osmolality to 341 ± 5 mosmol (kg water)-1 and elicited an immediate increase in PNA and tSNA (n = 6, P < 0.05) in sham-denervated rats. Carotid body removal attenuated sympathoexcitation (n = 5, P < 0.05) but did not affect the tachypnoeic response. A precollicular transection disconnecting the hypothalamus abolished the sympathoexcitatory and tachypnoeic responses to NaCl overload (n = 6, P < 0.05). Equi-osmolar infusions of mannitol did not alter the PNA and tSNA in sham-denervated rats (n = 5). Sodium chloride infusions increased carotid sinus nerve activity (n = 10, P < 0.05), whereas mannitol produced negligible changes (n = 5). The results indicate that carotid bodies are activated by acute NaCl overload, but not by mannitol. We conclude that the carotid bodies contribute to the increased sympathetic activity during acute NaCl overload, whereas the ventilatory response is mainly mediated by hypothalamic mechanisms.
Keywords: carotid sinus nerve; hyperosmotic NaCl; sympathetic nerve activity.
© 2018 The Authors. Experimental Physiology © 2018 The Physiological Society.
Conflict of interest statement
COMPETING INTERESTS
None declared.
Figures





Similar articles
-
A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation.J Physiol. 2006 Oct 15;576(Pt 2):569-83. doi: 10.1113/jphysiol.2006.115766. Epub 2006 Jul 27. J Physiol. 2006. PMID: 16873404 Free PMC article.
-
NaCl and osmolarity produce different responses in organum vasculosum of the lamina terminalis neurons, sympathetic nerve activity and blood pressure.J Physiol. 2017 Sep 15;595(18):6187-6201. doi: 10.1113/JP274537. Epub 2017 Aug 2. J Physiol. 2017. PMID: 28678348 Free PMC article.
-
AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation.Am J Physiol Regul Integr Comp Physiol. 2001 Dec;281(6):R1844-53. doi: 10.1152/ajpregu.2001.281.6.R1844. Am J Physiol Regul Integr Comp Physiol. 2001. PMID: 11705769
-
Translation of salt retention to central activation of the sympathetic nervous system in hypertension.Clin Exp Pharmacol Physiol. 2005 May-Jun;32(5-6):426-32. doi: 10.1111/j.1440-1681.2005.04206.x. Clin Exp Pharmacol Physiol. 2005. PMID: 15854153 Review.
-
Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease.J Physiol. 2010 Sep 15;588(Pt 18):3375-84. doi: 10.1113/jphysiol.2010.191940. Epub 2010 Jul 5. J Physiol. 2010. PMID: 20603334 Free PMC article. Review.
Cited by
-
Vasopressin and Breathing: Review of Evidence for Respiratory Effects of the Antidiuretic Hormone.Front Physiol. 2021 Oct 26;12:744177. doi: 10.3389/fphys.2021.744177. eCollection 2021. Front Physiol. 2021. PMID: 34867449 Free PMC article. Review.
-
The carotid body: A novel key player in neuroimmune interactions.Front Immunol. 2022 Oct 24;13:1033774. doi: 10.3389/fimmu.2022.1033774. eCollection 2022. Front Immunol. 2022. PMID: 36389846 Free PMC article. Review.
-
Effects of Voluntary Sodium Consumption during the Perinatal Period on Renal Mechanisms, Blood Pressure, and Vasopressin Responses after an Osmotic Challenge in Rats.Nutrients. 2023 Jan 4;15(2):254. doi: 10.3390/nu15020254. Nutrients. 2023. PMID: 36678125 Free PMC article.
-
Medullary Noradrenergic Neurons Mediate Hemodynamic Responses to Osmotic and Volume Challenges.Front Physiol. 2021 Apr 23;12:649535. doi: 10.3389/fphys.2021.649535. eCollection 2021. Front Physiol. 2021. PMID: 33967822 Free PMC article.
-
Hypoxia sensing in the body: An update on the peripheral and central mechanisms.Exp Physiol. 2024 Apr;109(4):461-469. doi: 10.1113/EP091206. Epub 2023 Nov 30. Exp Physiol. 2024. PMID: 38031809 Free PMC article. Review.
References
-
- Aicher SA, Saravay RH, Cravo S, Jeske I, Morrison SF, Reis DJ, & Milner TA (1996). Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: Comparison with input from the caudal ventrolateral medulla. The Journal of Comparative Neurology, 373, 62–75. - PubMed
-
- Amin MS, Wang HW, Reza E, Whitman SC, Tuana BS, & Leenen FH (2005). Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. American Journal Physiology. Regulatory, Integrative and Comparative Physiology, 289, R1787–R1797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

