Immune-Mediated Myocarditis in Fabry Disease Cardiomyopathy
- PMID: 30371172
- PMCID: PMC6201436
- DOI: 10.1161/JAHA.118.009052
Immune-Mediated Myocarditis in Fabry Disease Cardiomyopathy
Abstract
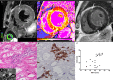
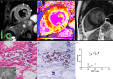
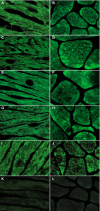
Background Glycosphingolipid accumulation in Fabry cells generates a proinflammatory response that may influence disease evolution and responsiveness to enzyme replacement therapy. This study evaluated incidence, mechanism, and impact of myocarditis in Fabry disease cardiomyopathy ( FDCM ). Methods and Results Myocarditis, defined as CD 3+ T lymphocytes >7/mm2 associated with necrosis of glycolipid-laden myocardiocytes, was retrospectively evaluated in endomyocardial biopsies from 78 patients with FDCM : 13 with maximal wall thickness (MWT) <11 mm (group 1), 17 with MWT 11 to 15 mm (group 2), 30 with MWT 16 to 20 mm (group 3), and 18 with MWT >20 mm (group 4). Myocarditis was investigated by polymerase chain reaction for cardiotropic viruses, by serum antiheart and antimyosin antibodies, and by cardiac magnetic resonance. Myocarditis was recognized at histology in 48 of 78 patients with FDCM (38% of group 1, 41% of group 2, 66% of group 3, and 72% of group 4). Myocarditis was characterized by positive antiheart and antimyosin antibodies and negative polymerase chain reaction for viral genomes. CD 3+ cells/mm2 correlated with myocyte necrosis, antimyosin autoantibody titer, and MWT ( P<0.001, r=0.79; P<0.001, r=0.84; P<0.001, r=0.61, respectively). Cardiac magnetic resonance showed myocardial edema in 24 of 78 patients (31%): 0% of group 1, 23% of group 2, 37% of group 3, and 50% of group 4. Conclusions Myocarditis is detectable at histology in up to 56% of patients with FDCM . It is immune mediated and correlates with disease severity. It can be disclosed by antiheart/antimyosin autoantibodies and in the advanced phase by cardiac magnetic resonance. It may contribute to progression of FDCM and resistance to enzyme replacement therapy.
Keywords: Fabry disease; cardiomyopathy; heart failure; myocarditis.
Figures




References
-
- Desnick RJ, Ioannou YA, Eng CM. Alpha‐galactosidase a deficiency: Fabry disease In: Scriver CR, Beaudet Al, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease. New York, NY: McGraw‐Hall; 2001:3733–3774.
-
- Desnick RJ. Fabry's disease (αGalactosidase A Deficiency): an X‐linked Nephropathy In: Lifton R, Somlo S, Giebisch, G, Seldin D, eds. Genetic Diseases of the Kidney. San Diego, CA: Elsevier Academic Press; 2009:597–616.
-
- Thurberg BL, Fallon JT, Mitchell R, Aretz T, Gordon RE, O'Callaghan MW. Cardiac microvascular pathology in Fabry disease: evaluation of endomyocardial biopsies before and after enzyme replacement therapy. Circulation. 2009;119:2561–2567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

