Discordant Alternans as a Mechanism for Initiation of Ventricular Fibrillation In Vitro
- PMID: 30371176
- PMCID: PMC6201417
- DOI: 10.1161/JAHA.117.007898
Discordant Alternans as a Mechanism for Initiation of Ventricular Fibrillation In Vitro
Abstract
Background Ventricular tachyarrhythmias are often preceded by short sequences of premature ventricular complexes. In a previous study, a restitution-based computational model predicted which sequences of stimulated premature complexes were most likely to induce ventricular fibrillation in canines in vivo. However, the underlying mechanism, based on discordant-alternans dynamics, could not be verified in that study. The current study seeks to elucidate the mechanism by determining whether the spatiotemporal evolution of action potentials and initiation of ventricular fibrillation in in vitro experiments are consistent with model predictions. Methods and Results Optical mapping voltage signals from canine right-ventricular tissue (n=9) were obtained simultaneously from the entire epicardium and endocardium during and after premature stimulus sequences. Model predictions of action potential propagation along a 1-dimensional cable were developed using action potential duration versus diastolic interval data. The model predicted sign-change patterns in action potential duration and diastolic interval spatial gradients with posterior probabilities of 91.1%, and 82.1%, respectively. The model predicted conduction block with 64% sensitivity and 100% specificity. A generalized estimating equation logistic-regression approach showed that model-prediction effects were significant for both conduction block ( P<1×10-15, coefficient 44.36) and sustained ventricular fibrillation ( P=0.0046, coefficient, 1.63) events. Conclusions The observed sign-change patterns favored discordant alternans, and the model successfully identified sequences of premature stimuli that induced conduction block. This suggests that the relatively simple discordant-alternans-based process that led to block in the model may often be responsible for ventricular fibrillation onset when preceded by premature beats. These observations may aid in developing improved methods for anticipating block and ventricular fibrillation.
Keywords: alternans; arrhythmia (mechanisms); computer‐based model; electrophysiology; ventricular fibrillation.
Figures
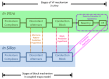
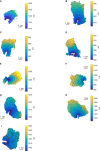


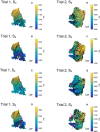


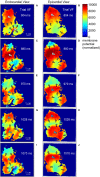
References
-
- Teodorescu C, Reinier K, Dervan C, Uy‐Evanado A, Samara M, Mariani R, Gunson K, Jui J, Chugh SS. Factors associated with pulseless electric activity versus ventricular fibrillation: the Oregon sudden unexpected death study. Circulation. 2010;122:2116–2122. - PubMed
-
- Kay GN, Plumb VJ, Arciniegas JG, Henthorn RW, Waldo AL. Torsade de pointes: the long‐short initiating sequence and other clinical features: observations in 32 patients. J Am Coll Cardiol. 1983;2:806–817. - PubMed
-
- Gomes JA, Alexopoulos D, Winters SL, Deshmukh P, Fuster V, Suh K. The role of silent ischemia, the arrhythmic substrate and the short‐long sequence in the genesis of sudden cardiac death. J Am Coll Cardiol. 1989;14:1618–1625. - PubMed
-
- el‐Sherif N, Gough WB, Restivo M. Reentrant ventricular arrhythmias in the late myocardial infarction period: mechanism by which a short‐long‐short cardiac sequence facilitates the induction of reentry. Circulation. 1991;83:268–278. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

