Calorie Restriction Curbs Proinflammation That Accompanies Arterial Aging, Preserving a Youthful Phenotype
- PMID: 30371211
- PMCID: PMC6222931
- DOI: 10.1161/JAHA.118.009112
Calorie Restriction Curbs Proinflammation That Accompanies Arterial Aging, Preserving a Youthful Phenotype
Erratum in
-
Calorie Restriction Curbs Proinflammation That Accompanies Arterial Aging, Preserving a Youthful Phenotype.J Am Heart Assoc. 2018 Nov 20;7(22):e004303. doi: 10.1161/JAHA.117.004303. J Am Heart Assoc. 2018. PMID: 30520335 Free PMC article. No abstract available.
Abstract
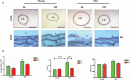
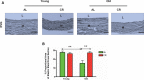
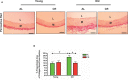
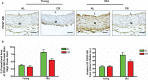
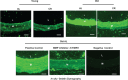
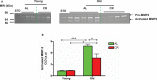
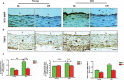
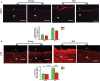
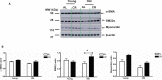
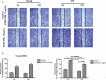
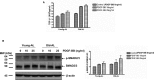
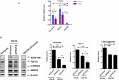
Background Aging exponentially increases the incidence of morbidity and mortality of quintessential cardiovascular disease mainly due to arterial proinflammatory shifts at the molecular, cellular, and tissue levels within the arterial wall. Calorie restriction ( CR ) in rats improves arterial function and extends both health span and life span. How CR affects the proinflammatory landscape of molecular, cellular, and tissue phenotypic shifts within the arterial wall in rats, however, remains to be elucidated. Methods and Results Aortae were harvested from young (6-month-old) and old (24-month-old) Fischer 344 rats, fed ad libitum and a second group maintained on a 40% CR beginning at 1 month of age. Histopathologic and morphometric analysis of the arterial wall demonstrated that CR markedly reduced age-associated intimal medial thickening, collagen deposition, and elastin fractionation/degradation within the arterial walls. Immunostaining/blotting showed that CR effectively prevented an age-associated increase in the density of platelet-derived growth factor, matrix metalloproteinase type II activity, and transforming growth factor beta 1 and its downstream signaling molecules, phospho-mothers against decapentaplegic homolog-2/3 (p- SMAD -2/3) in the arterial wall. In early passage cultured vascular smooth muscle cells isolated from AL and CR rat aortae, CR alleviated the age-associated vascular smooth muscle cell phenotypic shifts, profibrogenic signaling, and migration/proliferation in response to platelet-derived growth factor. Conclusions CR reduces matrix and cellular proinflammation associated with aging that occurs within the aortic wall and that are attributable to platelet-derived growth factor signaling. Thus, CR reduces the platelet-derived growth factor-associated signaling cascade, contributing to the postponement of biological aging and preservation of a more youthful aortic wall phenotype.
Keywords: aging; arterial remodeling; calorie restriction; proinflammation; rats; vascular smooth muscle cells.
Figures



Similar articles
-
Proinflammation, profibrosis, and arterial aging.Aging Med (Milton). 2020 Mar 18;3(3):159-168. doi: 10.1002/agm2.12099. eCollection 2020 Sep. Aging Med (Milton). 2020. PMID: 33103036 Free PMC article. Review.
-
Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways.Hypertension. 2010 Jun;55(6):1476-83. doi: 10.1161/HYPERTENSIONAHA.109.148783. Epub 2010 Apr 26. Hypertension. 2010. PMID: 20421514 Free PMC article.
-
Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall.Arterioscler Thromb Vasc Biol. 2006 Jul;26(7):1503-9. doi: 10.1161/01.ATV.0000225777.58488.f2. Epub 2006 May 11. Arterioscler Thromb Vasc Biol. 2006. PMID: 16690877
-
Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure.Hypertension. 2012 Aug;60(2):459-66. doi: 10.1161/HYPERTENSIONAHA.112.191270. Epub 2012 Jun 11. Hypertension. 2012. PMID: 22689745 Free PMC article.
-
Proinflammatory Arterial Stiffness Syndrome: A Signature of Large Arterial Aging.J Vasc Res. 2018;55(4):210-223. doi: 10.1159/000490244. Epub 2018 Aug 2. J Vasc Res. 2018. PMID: 30071538 Free PMC article. Review.
Cited by
-
Aging, oxidative stress and degenerative diseases: mechanisms, complications and emerging therapeutic strategies.Biogerontology. 2023 Oct;24(5):609-662. doi: 10.1007/s10522-023-10050-1. Epub 2023 Jul 30. Biogerontology. 2023. PMID: 37516673 Review.
-
Proinflammation, profibrosis, and arterial aging.Aging Med (Milton). 2020 Mar 18;3(3):159-168. doi: 10.1002/agm2.12099. eCollection 2020 Sep. Aging Med (Milton). 2020. PMID: 33103036 Free PMC article. Review.
-
Caloric restriction, Sirtuins, and cardiovascular diseases.Chin Med J (Engl). 2024 Apr 20;137(8):921-935. doi: 10.1097/CM9.0000000000003056. Epub 2024 Mar 25. Chin Med J (Engl). 2024. PMID: 38527930 Free PMC article. Review.
-
Accelerated elastin degradation by age-disease interaction: a common feature in age-related diseases.NPJ Aging. 2024 Feb 27;10(1):15. doi: 10.1038/s41514-024-00143-7. NPJ Aging. 2024. PMID: 38413600 Free PMC article.
-
Impact of Non-Pharmacological Interventions on the Mechanisms of Atherosclerosis.Int J Mol Sci. 2022 Aug 13;23(16):9097. doi: 10.3390/ijms23169097. Int J Mol Sci. 2022. PMID: 36012362 Free PMC article. Review.
References
-
- Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor‐beta1 (TGF‐beta1) and TGF‐beta1‐type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–1509. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

