Trastuzumab in Female Breast Cancer Patients With Reduced Left Ventricular Ejection Fraction
- PMID: 30371238
- PMCID: PMC6201446
- DOI: 10.1161/JAHA.118.008637
Trastuzumab in Female Breast Cancer Patients With Reduced Left Ventricular Ejection Fraction
Abstract
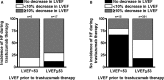
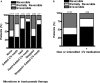
Background Trastuzumab is life-extending therapy for breast cancer patients overexpressing the human epidermal growth factor receptor 2 ( HER 2+), but has known cardiotoxic risk. We sought to determine if trastuzumab can be administered to patients with reduced baseline cardiac function at no higher cardiotoxicity risk than in those with normal cardiac function at baseline. Methods and Results We performed a retrospective study of women treated with trastuzumab for human epidermal growth factor receptor 2 breast cancer at Mayo Clinic Rochester between January 1, 2000 and August 31, 2015 with pre- and on-therapy echocardiograms available for review. A left ventricular ejection fraction (LVEF) <53% was considered abnormal, and a ≥10% decline in LVEF as evidence of cardiotoxicity based on the criteria of the American Society of Echocardiography. A total of 428 women were identified; 408 had a normal cardiac function ( LVEF 63.4±5%) and 20 had an impaired cardiac function ( LVEF 45.4±7%) before trastuzumab. Seven women (35%) with reduced LVEF at baseline had a ≥10% reduction in LVEF , compared with 179 (43.9%) of those with normal LVEF before trastuzumab initiation ( P= NS ). Symptomatic heart failure developed more often in patients with reduced versus normal baseline LVEF (25% versus 4.2%, P<0.05). After adjusting for patient age and breast cancer disease stage, survival rates over 5 years from time of diagnosis were found to be lower for patients with reduced baseline LVEF compared with patients with normal baseline LVEF ( P<0.001); the adjusted proportion of patients surviving at 5 years for those with low LVEF at baseline was 79% and for those with normal LVEF was 93%. Conclusions Women undergoing trastuzumab therapy for breast cancer with impaired baseline cardiac function experience no higher risk of LVEF decline, but more frequently develop symptomatic heart failure. While trastuzumab could be considered, these patients should be co-managed by a cardiologist.
Keywords: HER2; breast cancer; cardiomyopathy; cardiotoxicity; chemotherapy; heart failure; left ventricular ejection fraction; trastuzumab; treatment.
Figures

References
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
-
- Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the Her‐2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang C‐S, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Sütő T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
-
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti‐her2 monoclonal antibody in women who have her2‐overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

