Randomized Placebo-Controlled Trial Assessing the Effect of 24-Week Fenofibrate Therapy on Circulating Markers of Abdominal Aortic Aneurysm: Outcomes From the FAME -2 Trial
- PMID: 30371299
- PMCID: PMC6404864
- DOI: 10.1161/JAHA.118.009866
Randomized Placebo-Controlled Trial Assessing the Effect of 24-Week Fenofibrate Therapy on Circulating Markers of Abdominal Aortic Aneurysm: Outcomes From the FAME -2 Trial
Abstract
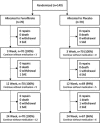
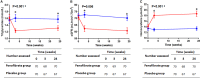
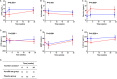
Background There is no drug therapy for abdominal aortic aneurysm ( AAA ). FAME-2 (Fenofibrate in the Management of Abdominal Aortic Aneurysm 2) was a placebo-controlled randomized trial designed to assess whether administration of 145 mg of fenofibrate/d for 24 weeks favorably modified circulating markers of AAA. Methods and Results Patients with AAA s measuring 35 to 49 mm and no contraindication were randomized to fenofibrate or identical placebo. The primary outcome measures were the differences in serum osteopontin and kallistatin concentrations between groups. Secondary analyses compared changes in the circulating concentration of AAA -associated proteins, and AAA growth, between groups using multivariable linear mixed-effects modeling. A total of 140 patients were randomized to receive fenofibrate (n=70) or placebo (n=70). By the end of the study 3 (2.1%) patients were lost to follow-up and 18 (12.9%) patients had ceased trial medication. A total of 85% of randomized patients took ≥80% of allocated tablets and were deemed to have complied with the medication regimen. Patients' allocated fenofibrate had expected reductions in serum triglycerides and estimated glomerular filtration rate, and increases in serum homocysteine. No differences in serum osteopontin, kallistatin, or AAA growth were observed between groups. Conclusions Administering 145 mg/d of fenofibrate for 24 weeks did not significantly reduce serum concentrations of osteopontin and kallistatin concentrations, or rates of AAA growth in this trial. The findings do not support the likely benefit of fenofibrate as a treatment for patients with small AAA s. Clinical Trial Registration URL : www.anzctr.org.au . Unique identifier: ACTRN 12613001039774.
Keywords: Fenofibrate; abdominal aortic aneurysm; biomarkers; clinical trial.
Figures


Comment in
-
Nascent Medical Therapies for Abdominal Aneurysms.J Am Heart Assoc. 2018 Oct 2;7(19):e010458. doi: 10.1161/JAHA.118.010458. J Am Heart Assoc. 2018. PMID: 30371312 Free PMC article. No abstract available.
References
-
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126:441–449. - PubMed
-
- Wanhainen A, Hultgren R, Linne A, Holst J, Gottsater A, Langenskiold M, Smidfelt K, Bjorck M, Svensjo S. Outcome of the Swedish nationwide abdominal aortic aneurysm screening program. Circulation. 2016;134:1141–1148. - PubMed
-
- Svensjo S, Mani K, Bjorck M, Lundkvist J, Wanhainen A. Screening for abdominal aortic aneurysm in 65‐year‐old men remains cost‐effective with contemporary epidemiology and management. Eur J Vasc Endovasc Surg. 2014;47:357–365. - PubMed
-
- Khosla S, Morris DR, Moxon JV, Walker PJ, Gasser T, Golledge J. Meta‐analysis of peak wall stress in ruptured, symptomatic and intact abdominal aortic aneurysms. Br J Surg. 2014;101:1350–1357. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

