Effect of Once-Weekly Exenatide on Clinical Outcomes According to Baseline Risk in Patients With Type 2 Diabetes Mellitus: Insights From the EXSCEL Trial
- PMID: 30371301
- PMCID: PMC6404902
- DOI: 10.1161/JAHA.118.009304
Effect of Once-Weekly Exenatide on Clinical Outcomes According to Baseline Risk in Patients With Type 2 Diabetes Mellitus: Insights From the EXSCEL Trial
Abstract
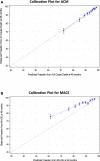
Background In the EXSCEL (Exenatide Study of Cardiovascular Event Lowering), exenatide once-weekly resulted in a nonsignificant reduction in major adverse cardiovascular events ( MACEs ) and a nominal 14% reduction in all-cause mortality in 14 752 patients with type 2 diabetes mellitus (T2 DM ) with and without cardiovascular disease. Whether patients at increased risk for events experienced a comparatively greater treatment benefit with exenatide is unknown. Methods and Results In the EXSCEL population, we created risk scores for MACEs and all-cause mortality using step-wise selection of baseline characteristics. A risk score was calculated for each patient, and a time-to-event model for each end point was developed including the risk score, treatment assignment, and risk-treatment interaction. Interaction P values evaluating for a differential treatment effect by baseline risk were reported. Over a median follow-up of 3.2 years (interquartile range, 2.2, 4.4), 1091 (7.4%) patients died and 1744 (11.8%) experienced a MACE . Independent predictors of MACEs and all-cause mortality included age, sex, comorbidities (eg, previous cardiovascular event), body mass index, blood pressure, hemoglobin A1c, and estimated glomerular filtration rate. The all-cause mortality and MACE risk models had modest discrimination with optimism-corrected c-indices of 0.73 and 0.71, respectively. No interaction was observed between treatment effect and risk profile for either end point (both interactions, P>0.1). Conclusions Baseline characteristics (eg, age, previous cardiovascular events) and routine laboratory values (eg, hemoglobin A1c, estimated glomerular filtration rate) provided modest prognostic value for mortality and MACEs in a broad population of patients with type 2 diabetes mellitus. Exenatide's effects on mortality and MACEs were consistent across the spectrum of baseline risk. Clinical Trial Registration URL: https://www.clinicaltrials.gov . Unique identifier: NCT 01144338.
Trial registration: ClinicalTrials.gov NCT01144338.
Keywords: glucagon‐like peptide‐1 receptor agonis; major adverse cardiac event; mortality; type 2 diabetes mellitus.
Figures


References
-
- Holman RR, Bethel MA, George J, Sourij H, Doran Z, Keenan J, Khurmi NS, Mentz RJ, Oulhaj A, Buse JB, Chan JC, Iqbal N, Kundu S, Maggioni AP, Marso SP, Ohman P, Pencina MJ, Poulter N, Porter LE, Ramachandran A, Zinman B, Hernandez AF. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103–110. - PubMed
-
- Mentz RJ, Bethel MA, Gustavson S, Thompson VP, Pagidipati NJ, Buse JB, Chan JC, Iqbal N, Maggioni AP, Marso SP, Ohman P, Poulter N, Ramachandran A, Zinman B, Hernandez AF, Holman RR. Baseline characteristics of patients enrolled in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL). Am Heart J. 2017;187:1–9. - PMC - PubMed
-
- Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Ohman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. - PMC - PubMed
-
- Drucker DJ. The cardiovascular biology of glucagon‐like peptide‐1. Cell Metab. 2016;24:15–30. - PubMed
-
- Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. - PubMed

