Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated With Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study
- PMID: 30371304
- PMCID: PMC6404886
- DOI: 10.1161/JAHA.118.009961
Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated With Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study
Abstract
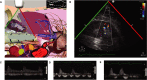
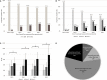
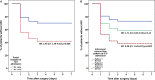
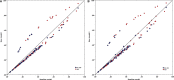
Background Acute kidney injury ( AKI ) after cardiac surgery is associated with adverse outcomes. Venous congestion can impair kidney function, but few tools are available to assess its impact at the bedside. The objective of this study was to determine whether portal flow pulsatility and alterations in intrarenal venous flow assessed by Point-Of-Care ultrasound are associated with AKI after cardiac surgery. Methods and Results This single-center prospective cohort study recruited patients undergoing cardiac surgery with cardiopulmonary bypass. Hepatic and renal Doppler ultrasound assessments were performed before surgery, at the intensive care unit admission, and daily for 3 days after surgery. The primary statistical analysis was performed using proportional hazards model for time-dependent variables. Among the 145 patients included, 49 patients (33.8%) developed AKI after cardiac surgery. The detection of portal flow pulsatility was associated with an increased risk of AKI (hazard ratio: 2.09, confidence interval, 1.11-3.94, P=0.02), as were severe alterations of intrarenal venous flow (hazard ratio: 2.81, confidence interval, 1.42-5.56, P=0.003). These associations remained significant in multivariable models. The addition of these markers to preoperative risk factors and central venous pressure measurement at intensive care unit admission improved the prediction of AKI . (Continuous net reclassification improvement: 0.364, confidence interval, 0.081-0.652 for portal Doppler and net reclassification improvement: 0.343, confidence interval, 0.081-0.628 for intrarenal Doppler) Conclusions Portal flow pulsatility and intrarenal flow alterations are markers of venous congestion and are independently associated with AKI after cardiac surgery. These tools might offer valuable information to develop strategies aimed at treating or preventing congestive cardiorenal syndrome after cardiac surgery. Clinical Trial Registration URL : https://www.clinicaltrials.gov . Unique identifier: NCT 02831907.
Keywords: acute kidney injury; cardiac surgery; congestive heart failure; point‐of‐care ultrasound.
Figures


References
-
- Wang Y, Bellomo R. Cardiac surgery‐associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13:697–711. - PubMed
-
- Styczynski G, Milewska A, Marczewska M, Sobieraj P, Sobczynska M, Dabrowski M, Kuch‐Wocial A, Szmigielski C. Echocardiographic correlates of abnormal liver tests in patients with exacerbation of chronic heart failure. J Am Soc Echocardiogr. 2016;29:132–139. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

