Viscoelastic Properties of Human Autopsy Brain Tissues as Biomarkers for Alzheimer's Diseases
- PMID: 30371351
- PMCID: PMC6605047
- DOI: 10.1109/TBME.2018.2878555
Viscoelastic Properties of Human Autopsy Brain Tissues as Biomarkers for Alzheimer's Diseases
Abstract
Objective: The present study investigates viscoelastic properties of human autopsy brain tissue via nanoindentation to find feasible biomarkers for Alzheimer's disease (AD) in ex vivo condition and to understand the mechanics of the human brain better, especially on the difference before and after progression of AD.
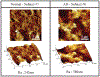
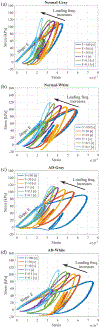
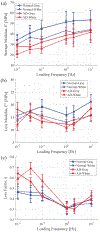
Methods: Viscoelastic properties of paraformaldehyde-fixed, paraffin-embedded thin (8 [Formula: see text]) sectioned normal and AD affected human autopsy brain tissue samples are investigated via nanoindentation with a combined loading profile of a linear preloading and a sinusoidal loading at various loading frequencies from 0.01 to 10 [Formula: see text]. In 1200 indentation tests for ten human autopsy brain tissue samples from ten different subjects (five AD cases and five normal controls), viscoelastic properties such as Young's modulus, storage modulus, loss modulus, and loss factor of both gray and white matter brain tissues samples from normal and AD affected tissues were measured experimentally.
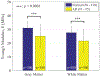
Results: We found that the normal brain tissues have higher Young's modulus values than the AD affected brain tissues by 23.5 % and 27.9 % on average for gray and white matter, respectively, with statistically significant differences ( ) between the normal and AD affected brain tissues. Additionally, the AD affected brain tissues have much higher loss factor than the normal brain tissues on lower loading frequencies.
Significance: AD is one of the leading causes of death in America and continues to affect a growing population. The challenges of recognizing the early pathological changes in brain tissue due to AD and diagnosing a patient has led to much research focused on finding biomarkers for the disease. In this regard, understanding the mechanics of brain tissues is increasingly recognized to play an important role in diagnosing brain diseases.
Figures



Similar articles
-
Longitudinal structural cerebral changes related to core CSF biomarkers in preclinical Alzheimer's disease: A study of two independent datasets.Neuroimage Clin. 2018 Apr 16;19:190-201. doi: 10.1016/j.nicl.2018.04.016. eCollection 2018. Neuroimage Clin. 2018. PMID: 30023169 Free PMC article.
-
Examination of Alzheimer's disease by a combination of electrostatic force and mechanical measurement.J Microsc. 2019 Jul;275(1):66-72. doi: 10.1111/jmi.12801. Epub 2019 May 9. J Microsc. 2019. PMID: 31038737
-
Characterization of the linearly viscoelastic behavior of human tympanic membrane by nanoindentation.J Mech Behav Biomed Mater. 2009 Jan;2(1):82-92. doi: 10.1016/j.jmbbm.2008.05.008. Epub 2008 Jun 12. J Mech Behav Biomed Mater. 2009. PMID: 19627811
-
Quantitative MRI to understand Alzheimer's disease pathophysiology.Curr Opin Neurol. 2016 Aug;29(4):437-44. doi: 10.1097/WCO.0000000000000345. Curr Opin Neurol. 2016. PMID: 27228309 Review.
-
Mapping cellular transcriptosomes in autopsied Alzheimer's disease subjects and relevant animal models.Neurobiol Aging. 2006 Aug;27(8):1060-77. doi: 10.1016/j.neurobiolaging.2005.04.014. Epub 2005 Sep 12. Neurobiol Aging. 2006. PMID: 16157420 Review.
Cited by
-
Quantitative Phase Imaging: Recent Advances and Expanding Potential in Biomedicine.ACS Nano. 2022 Aug 23;16(8):11516-11544. doi: 10.1021/acsnano.1c11507. Epub 2022 Aug 2. ACS Nano. 2022. PMID: 35916417 Free PMC article. Review.
-
Unraveling the Local Relation Between Tissue Composition and Human Brain Mechanics Through Machine Learning.Front Bioeng Biotechnol. 2021 Aug 17;9:704738. doi: 10.3389/fbioe.2021.704738. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34485258 Free PMC article.
-
Meta-analysis of the make-up and properties of in vitro models of the healthy and diseased blood-brain barrier.Nat Biomed Eng. 2025 Apr;9(4):566-598. doi: 10.1038/s41551-024-01250-2. Epub 2024 Sep 20. Nat Biomed Eng. 2025. PMID: 39304761
-
Vesicle fusion and release in neurons under dynamic mechanical equilibrium.iScience. 2024 Apr 19;27(5):109793. doi: 10.1016/j.isci.2024.109793. eCollection 2024 May 17. iScience. 2024. PMID: 38736547 Free PMC article. Review.
-
Photoacoustic viscoelasticity assessment of prefrontal cortex and cerebellum in normal and prenatal valproic acid-exposed rats.Photoacoustics. 2024 Jan 20;36:100590. doi: 10.1016/j.pacs.2024.100590. eCollection 2024 Apr. Photoacoustics. 2024. PMID: 38318427 Free PMC article.
References
-
- Dubois B et al., “Advancing Research Diagnostic Criteria for Alzheimer’s Disease: The IWG-2 Criteria,” Lancet Neurol., vol. 13, no. 6, pp. 614–629, 2014. - PubMed
-
- Alzheimer’s Association, “2018 Alzheimer’s Disease Facts and Figures,” Alzheimers Dement., vol. 14, no. 3, pp. 367–429, 2018.
-
- Prince M et al., “World Alzheimer Report 2016 - Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future.” London, UK: Alzheimer’s Disease International (ADI), 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

