Influenza Vaccine Effectiveness and Statin Use Among Adults in the United States, 2011-2017
- PMID: 30371753
- PMCID: PMC6495015
- DOI: 10.1093/cid/ciy780
Influenza Vaccine Effectiveness and Statin Use Among Adults in the United States, 2011-2017
Abstract
Background: Statin medications have immunomodulatory effects. Several recent studies suggest that statins may reduce influenza vaccine response and reduce influenza vaccine effectiveness (VE).
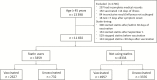
Methods: We compared influenza VE in statin users and nonusers aged ≥45 years enrolled in the US Vaccine Effectiveness Network study over 6 influenza seasons (2011-2012 through 2016-2017). All enrollees presented to outpatients clinics with acute respiratory illness and were tested for influenza. Information on vaccination status, medical history, and statin use at the time of vaccination were collected by medical and pharmacy records. Using a test-negative design, we estimated VE as (1 - OR) × 100, in which OR is the odds ratio for testing positive for influenza virus among vaccinated vs unvaccinated participants.
Results: Among 11692 eligible participants, 3359 (30%) were statin users and 2806 (24%) tested positive for influenza virus infection; 78% of statin users and 60% of nonusers had received influenza vaccine. After adjusting for potential confounders, influenza VE was 36% (95% confidence interval [CI], 22%-47%) among statin users and 39% (95% CI, 32%-45%) among nonusers. We observed no significant modification of VE by statin use. VE against influenza A(H1N1)pdm09, A(H3N2), and B viruses were similar among statin users and nonusers.
Conclusions: In this large observational study, influenza VE against laboratory-confirmed influenza illness was not affected by current statin use among persons aged ≥45 years. Statin use did not modify the effect of vaccination on influenza when analyzed by type and subtype.
Keywords: influenza vaccine; statins; vaccine effectiveness.
Published by Oxford University Press for the Infectious Diseases Society of America 2018.
Figures

References
-
- Darvishian M, Bijlsma MJ, Hak E, van den Heuvel ER. Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies. Lancet Infect Dis 2014; 14:1228–39. - PubMed
-
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159–69. - PubMed
-
- Gu Q, Paulose-Ram R, Burt VL, Kit BK. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. NCHS Data Brief 2014:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR024153/RR/NCRR NIH HHS/United States
- U01 IP001034/IP/NCIRD CDC HHS/United States
- U01 IP000474/IP/NCIRD CDC HHS/United States
- U01 IP000473/IP/NCIRD CDC HHS/United States
- U01 IP001039/IP/NCIRD CDC HHS/United States
- U01 IP000471/IP/NCIRD CDC HHS/United States
- U01 IP000467/IP/NCIRD CDC HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U01 IP000466/IP/NCIRD CDC HHS/United States
- U01 IP001035/IP/NCIRD CDC HHS/United States
- U01 IP001037/IP/NCIRD CDC HHS/United States
- U01 IP001038/IP/NCIRD CDC HHS/United States
LinkOut - more resources
Full Text Sources
Medical

