Candidate-based screening via gene modulation in human neurons and astrocytes implicates FERMT2 in Aβ and TAU proteostasis
- PMID: 30371777
- PMCID: PMC6381320
- DOI: 10.1093/hmg/ddy376
Candidate-based screening via gene modulation in human neurons and astrocytes implicates FERMT2 in Aβ and TAU proteostasis
Abstract
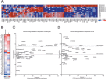
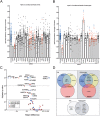
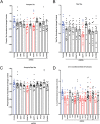
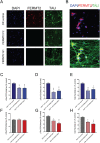
Large-scale 'omic' studies investigating the pathophysiological processes that lead to Alzheimer's disease (AD) dementia have identified an increasing number of susceptibility genes, many of which are poorly characterized and have not previously been implicated in AD. Here, we evaluated the utility of human induced pluripotent stem cell-derived neurons and astrocytes as tools to systematically test AD-relevant cellular phenotypes following perturbation of candidate genes identified by genome-wide studies. Lentiviral-mediated delivery of shRNAs was used to modulate expression of 66 genes in astrocytes and 52 genes in induced neurons. Five genes (CNN2, GBA, GSTP1, MINT2 and FERMT2) in neurons and nine genes (CNN2, ITGB1, MINT2, SORL1, VLDLR, NPC1, NPC2, PSAP and SCARB2) in astrocytes significantly altered extracellular amyloid-β (Aβ) levels. Knockdown of AP3M2, CNN2, GSTP1, NPC1, NPC2, PSAP and SORL1 reduced interleukin-6 levels in astrocytes. Only knockdown of FERMT2 led to a reduction in the proportion of TAU that is phosphorylated. Further, CRISPR-Cas9 targeting of FERMT2 in both familial AD (fAD) and fAD-corrected human neurons validated the findings of reduced extracellular Aβ. Interestingly, FERMT2 reduction had no effect on the Aβ42:40 ratio in corrected neurons and a reduction of phospho-tau, but resulted in an elevation in Aβ42:40 ratio and no reduction in phospho-tau in fAD neurons. Taken together, this study has prioritized 15 genes as being involved in contributing to Aβ accumulation, phosphorylation of tau and/or cytokine secretion, and, as illustrated with FERMT2, it sets the stage for further cell-type-specific dissection of the role of these genes in AD.
© The Author(s) 2018. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




Similar articles
-
Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation.Alzheimers Res Ther. 2017 Dec 1;9(1):90. doi: 10.1186/s13195-017-0317-z. Alzheimers Res Ther. 2017. PMID: 29191219 Free PMC article.
-
The participation of insulin-like growth factor-binding protein 3 released by astrocytes in the pathology of Alzheimer's disease.Mol Brain. 2015 Dec 4;8(1):82. doi: 10.1186/s13041-015-0174-2. Mol Brain. 2015. PMID: 26637371 Free PMC article.
-
Do astrocytes collaborate with neurons in spreading the "infectious" aβ and Tau drivers of Alzheimer's disease?Neuroscientist. 2015 Feb;21(1):9-29. doi: 10.1177/1073858414529828. Epub 2014 Apr 16. Neuroscientist. 2015. PMID: 24740577
-
Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer's Disease.Cells. 2019 May 22;8(5):488. doi: 10.3390/cells8050488. Cells. 2019. PMID: 31121890 Free PMC article. Review.
-
Key Peptides and Proteins in Alzheimer's Disease.Curr Protein Pept Sci. 2019;20(6):577-599. doi: 10.2174/1389203720666190103123434. Curr Protein Pept Sci. 2019. PMID: 30605056 Review.
Cited by
-
Relationship Between FERMT2, CELF1, COPI, CHRNA2, and ABCA7 Genetic Polymorphisms and Alzheimer's Disease Risk in the Southern Chinese Population.J Alzheimers Dis Rep. 2023 Nov 9;7(1):1247-1257. doi: 10.3233/ADR-230072. eCollection 2023. J Alzheimers Dis Rep. 2023. PMID: 38025799 Free PMC article.
-
Efficient manipulation of gene dosage in human iPSCs using CRISPR/Cas9 nickases.Commun Biol. 2021 Feb 12;4(1):195. doi: 10.1038/s42003-021-01722-0. Commun Biol. 2021. PMID: 33580208 Free PMC article.
-
The Rise of Pluripotent Stem Cell-Derived Glia Models of Neuroinflammation.Neurol Int. 2025 Jan 13;17(1):6. doi: 10.3390/neurolint17010006. Neurol Int. 2025. PMID: 39852770 Free PMC article. Review.
-
Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer's disease brain.J Extracell Vesicles. 2022 Jan;11(1):e12183. doi: 10.1002/jev2.12183. J Extracell Vesicles. 2022. PMID: 35029059 Free PMC article.
-
Predictive network analysis identifies JMJD6 and other potential key drivers in Alzheimer's disease.Commun Biol. 2023 May 15;6(1):503. doi: 10.1038/s42003-023-04791-5. Commun Biol. 2023. PMID: 37188718 Free PMC article.
References
-
- Bertram L., McQueen M.B., Mullin K., Blacker D. and Tanzi R.E. (2007) Systematic meta-analyses of Alzheimer's disease genetic association studies: the AlzGene database. Nat. Genet., 39, 17–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

