LncRNA-OG Promotes the Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells Under the Regulation of hnRNPK
- PMID: 30372559
- PMCID: PMC7379496
- DOI: 10.1002/stem.2937
LncRNA-OG Promotes the Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells Under the Regulation of hnRNPK
Abstract
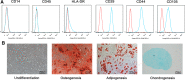
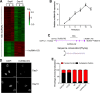
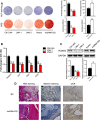
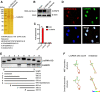
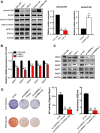
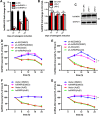
Bone marrow-derived mesenchymal stem cells (BM-MSCs) are the main source of osteoblasts in vivo and are widely used in stem cell therapy. Previously, we analyzed long noncoding RNA (lncRNA) expression profiles during BM-MSC osteogenesis, and further investigation is needed to elucidate how lncRNAs regulate BM-MSC osteogenesis. Herein, we used customized microarrays to determine lncRNA expression profiles in BM-MSCs on days 0 and 10 of osteogenic differentiation. In addition, we identified a novel osteogenesis-associated lncRNA (lncRNA-OG) that is upregulated during this process. Functional assays showed that lncRNA-OG significantly promotes BM-MSC osteogenesis. Mechanistically, lncRNA-OG interacts with heterogeneous nuclear ribonucleoprotein K (hnRNPK) protein to regulate bone morphogenetic protein signaling pathway activation. Surprisingly, hnRNPK positively regulates lncRNA-OG transcriptional activity by promoting H3K27 acetylation of the lncRNA-OG promoter. Therefore, our study revealed a novel lncRNA with a positive function on BM-MSC osteogenic differentiation and proposed a new interaction between hnRNPK and lncRNA. Stem Cells 2018 Stem Cells 2019;37:270-283.
Keywords: Heterogeneous nuclear ribonucleoprotein K; Long noncoding RNA; Mesenchymal stem cells; Osteogenic differentiation.
© 2018 The Authors. Stem Cells published by Wiley Periodicals, Inc. on behalf of AlphaMed Press 2018.
Conflict of interest statement
The authors indicated no potential conflicts of interest.
Figures

References
-
- Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol 2000;16:191–220. - PubMed
-
- Kronenberg HM. Developmental regulation of the growth plate. Nature 2003;423:332–336. - PubMed
-
- Xie Z, Wang P, Li Y et al. Imbalance between bone morphogenetic protein 2 and noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol 2016;68:430–440. - PubMed
-
- Lian WS, Wu RW, Lee MS et al. Subchondral mesenchymal stem cells from osteoarthritic knees display high osteogenic differentiation capacity through microRNA‐29a regulation of HDAC4. J Mol Med 2017;95:1327–1340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

