Constriction of Retinal Venules to Endothelin-1: Obligatory Roles of ETA Receptors, Extracellular Calcium Entry, and Rho Kinase
- PMID: 30372743
- PMCID: PMC6203175
- DOI: 10.1167/iovs.18-25369
Constriction of Retinal Venules to Endothelin-1: Obligatory Roles of ETA Receptors, Extracellular Calcium Entry, and Rho Kinase
Abstract
Purpose: Endothelin-1 (ET-1) is a potent vasoconstrictor peptide implicated in retinal venous pathologies such as diabetic retinopathy and retinal vein occlusion. However, underlying mechanisms contributing to venular constriction remain unknown. Thus, we examined the roles of ET-1 receptors, extracellular calcium (Ca2+), L-type voltage-operated calcium channels (L-VOCCs), Rho kinase (ROCK), and protein kinase C (PKC) in ET-1-induced constriction of retinal venules.
Methods: Porcine retinal venules were isolated and pressurized for vasoreactivity study using videomicroscopic techniques. Protein and mRNA were analyzed using molecular tools.
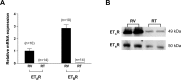
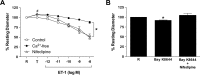
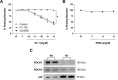
Results: Retinal venules developed basal tone and constricted concentration-dependently to ET-1. The ETA receptor (ETAR) antagonist BQ123 abolished venular constriction to ET-1, but ETB receptor (ETBR) antagonist BQ788 had no effect on vasoconstriction. The ETBR agonist sarafotoxin S6c did not elicit vasomotor activity. In the absence of extracellular Ca2+, venules lost basal tone and ET-1-induced constriction was nearly abolished. Although L-VOCC inhibitor nifedipine also reduced basal tone and blocked vasoconstriction to L-VOCC activator Bay K8644, constriction of venules to ET-1 remained. The ROCK inhibitor H-1152 but not PKC inhibitor Gö 6983 prevented ET-1-induced vasoconstriction. Protein and mRNA expressions of ETARs and ETBRs, along with ROCK1 and ROCK2 isoforms, were detected in retinal venules.
Conclusions: Extracellular Ca2+ entry via L-VOCCs is essential for developing and maintaining basal tone of porcine retinal venules. ET-1 causes significant constriction of retinal venules by activating ETARs and extracellular Ca2+ entry independent of L-VOCCs. Activation of ROCK signaling, without involvement of PKC, appears to mediate venular constriction to ET-1 in the porcine retina.
Figures

Similar articles
-
Constriction of retinal arterioles to endothelin-1: requisite role of rho kinase independent of protein kinase C and L-type calcium channels.Invest Ophthalmol Vis Sci. 2012 May 17;53(6):2904-12. doi: 10.1167/iovs.12-9542. Invest Ophthalmol Vis Sci. 2012. PMID: 22427601 Free PMC article.
-
Role of endothelium in vasomotor responses to endothelin system and protein kinase C activation in porcine retinal arterioles.Invest Ophthalmol Vis Sci. 2013 Nov 15;54(12):7587-94. doi: 10.1167/iovs13-13178. Invest Ophthalmol Vis Sci. 2013. PMID: 24243985 Free PMC article.
-
Functional and molecular characterization of the endothelin system in retinal arterioles.Invest Ophthalmol Vis Sci. 2009 Jul;50(7):3329-36. doi: 10.1167/iovs.08-3129. Epub 2009 Jan 17. Invest Ophthalmol Vis Sci. 2009. PMID: 19151386 Free PMC article.
-
Endothelin receptor signaling: new insight into its regulatory mechanisms.J Pharmacol Sci. 2013;123(2):85-101. doi: 10.1254/jphs.13r02cr. Epub 2013 Sep 27. J Pharmacol Sci. 2013. PMID: 24077109 Review.
-
Endothelin receptors and calcium signaling.FASEB J. 1995 Sep;9(12):1196-204. doi: 10.1096/fasebj.9.12.7672512. FASEB J. 1995. PMID: 7672512 Review.
Cited by
-
Rho-Kinase Inhibitors for the Treatment of Refractory Diabetic Macular Oedema.Cells. 2021 Jul 3;10(7):1683. doi: 10.3390/cells10071683. Cells. 2021. PMID: 34359853 Free PMC article. Review.
-
Association of Cardiovascular Disease with Retinopathy of Prematurity.Ophthalmic Epidemiol. 2023 Feb;30(1):95-102. doi: 10.1080/09286586.2022.2036766. Epub 2022 Feb 9. Ophthalmic Epidemiol. 2023. PMID: 35137647 Free PMC article.
-
Hyperglycemia Augments Endothelin-1-Induced Constriction of Human Retinal Venules.Transl Vis Sci Technol. 2020 Aug 3;9(9):1. doi: 10.1167/tvst.9.9.1. eCollection 2020 Aug. Transl Vis Sci Technol. 2020. PMID: 32879758 Free PMC article.
-
Sitting leg vasculopathy: potential adaptations beyond the endothelium.Am J Physiol Heart Circ Physiol. 2024 Mar 1;326(3):H760-H771. doi: 10.1152/ajpheart.00489.2023. Epub 2024 Jan 19. Am J Physiol Heart Circ Physiol. 2024. PMID: 38241008 Free PMC article. Review.
-
Reactive Oxygen Species Are Essential for Vasoconstriction upon Cold Exposure.Oxid Med Cell Longev. 2021 Nov 24;2021:8578452. doi: 10.1155/2021/8578452. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34868457 Free PMC article.
References
-
- Kiel JW. The Ocular Circulation 1st ed. San Rafael, CA: Morgan & Claypool Life Sciences; 2010. pp. 1–69. - PubMed
-
- Johnson PC. Overview of the microcirculation. In: Tuma RF, Duran WN, Ley K, editors. Handbook of Physiology: The Cardiovascular System, Microcirculation 2nd ed. San Diego, CA: Academic Press;; 2008. pp. xi–xxiv.
-
- Davis MJ, Hill MA, Kuo L. Local regulation of microvascular perfusion. In: Tuma RF, Duran WN, Ley K, editors. Handbook of Physiology: The Cardiovascular System, Microcirculation (Section 2) 2nd ed. San Diego, CA: Academic Press;; 2008. pp. 161–284.
-
- Ye XD, Laties AM, Stone RA. Peptidergic innervation of the retinal vasculature and optic nerve head. Invest Ophthalmol Vis Sci. 1990;31:1731–1737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

