Chemotherapy-Exacerbated Breast Cancer Metastasis: A Paradox Explainable by Dysregulated Adaptive-Response
- PMID: 30373101
- PMCID: PMC6274941
- DOI: 10.3390/ijms19113333
Chemotherapy-Exacerbated Breast Cancer Metastasis: A Paradox Explainable by Dysregulated Adaptive-Response
Abstract
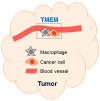
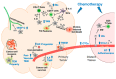
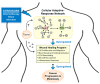
An emerging picture in cancer biology is that, paradoxically, chemotherapy can actively induce changes that favor cancer progression. These pro-cancer changes can be either inside (intrinsic) or outside (extrinsic) the cancer cells. In this review, we will discuss the extrinsic pro-cancer effect of chemotherapy; that is, the effect of chemotherapy on the non-cancer host cells to promote cancer progression. We will focus on metastasis, and will first discuss recent data from mouse models of breast cancer. Despite reducing the size of primary tumors, chemotherapy changes the tumor microenvironment, resulting in an increased escape of cancer cells into the blood stream. Furthermore, chemotherapry changes the tissue microenvironment at the distant sites, making it more hospitable to cancer cells upon their arrival. We will then discuss the idea and evidence that these devastating pro-metastatic effects of chemotherapy can be explained in the context of adaptive-response. At the end, we will discuss the potential relevance of these mouse data to human breast cancer and their implication on chemotherapy in the clinic.
Keywords: ATF3; adaptive-response network; breast cancer metastasis; cancer-host interaction; chemotherapy; immune modulation; seed and soil theory; stress response; tumor immune environment; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

