Recent Advances in Indazole-Containing Derivatives: Synthesis and Biological Perspectives
- PMID: 30373212
- PMCID: PMC6278422
- DOI: 10.3390/molecules23112783
Recent Advances in Indazole-Containing Derivatives: Synthesis and Biological Perspectives
Abstract
Indazole-containing derivatives represent one of the most important heterocycles in drug molecules. Diversely substituted indazole derivatives bear a variety of functional groups and display versatile biological activities; hence, they have gained considerable attention in the field of medicinal chemistry. This review aims to summarize the recent advances in various methods for the synthesis of indazole derivatives. The current developments in the biological activities of indazole-based compounds are also presented.
Keywords: biological activities; indazole; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


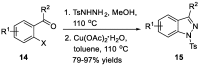
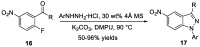
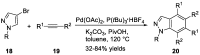
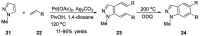


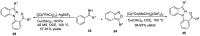

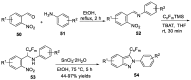
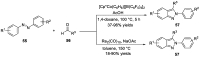


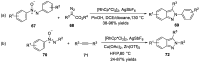


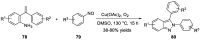







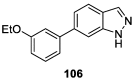

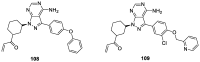

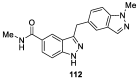

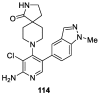


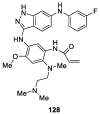
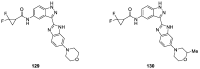

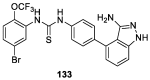


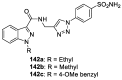









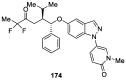
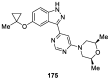
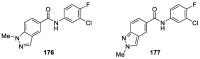
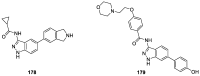






Similar articles
-
Synthesis, Antiprotozoal Activity, and Cheminformatic Analysis of 2-Phenyl-2H-Indazole Derivatives.Molecules. 2021 Apr 8;26(8):2145. doi: 10.3390/molecules26082145. Molecules. 2021. PMID: 33917871 Free PMC article.
-
Indazole Derivatives: Promising Anti-tumor Agents.Anticancer Agents Med Chem. 2018;18(9):1228-1234. doi: 10.2174/1871520618666180510113822. Anticancer Agents Med Chem. 2018. PMID: 29745343 Review.
-
Indazole and its Derivatives in Cardiovascular Diseases: Overview, Current Scenario, and Future Perspectives.Curr Top Med Chem. 2022;22(14):1177-1188. doi: 10.2174/1568026621666211214151534. Curr Top Med Chem. 2022. PMID: 34906057 Free PMC article. Review.
-
Indazole derivatives and their therapeutic applications: a patent review (2013-2017).Expert Opin Ther Pat. 2018 Jun;28(6):441-453. doi: 10.1080/13543776.2018.1472240. Epub 2018 May 11. Expert Opin Ther Pat. 2018. PMID: 29718740 Review.
-
Efficient MW-Assisted Synthesis, Spectroscopic Characterization, X-ray and Antioxidant Properties of Indazole Derivatives.Molecules. 2016 Jul 9;21(7):903. doi: 10.3390/molecules21070903. Molecules. 2016. PMID: 27409599 Free PMC article.
Cited by
-
A Suitable Functionalization of Nitroindazoles with Triazolyl and Pyrazolyl Moieties via Cycloaddition Reactions.Molecules. 2019 Dec 28;25(1):126. doi: 10.3390/molecules25010126. Molecules. 2019. PMID: 31905680 Free PMC article.
-
Synthesis, Antiprotozoal Activity, and Cheminformatic Analysis of 2-Phenyl-2H-Indazole Derivatives.Molecules. 2021 Apr 8;26(8):2145. doi: 10.3390/molecules26082145. Molecules. 2021. PMID: 33917871 Free PMC article.
-
Uniting Amide Synthesis and Activation by PIII/PV-Catalyzed Serial Condensation: Three-Component Assembly of 2-Amidopyridines.J Am Chem Soc. 2021 Sep 15;143(36):14487-14494. doi: 10.1021/jacs.1c07608. Epub 2021 Sep 3. J Am Chem Soc. 2021. PMID: 34478308 Free PMC article.
-
Regioselective N-alkylation of the 1H-indazole scaffold; ring substituent and N-alkylating reagent effects on regioisomeric distribution.Beilstein J Org Chem. 2021 Aug 2;17:1939-1951. doi: 10.3762/bjoc.17.127. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34386104 Free PMC article.
-
Rapid and halide compatible synthesis of 2-N-substituted indazolone derivatives via photochemical cyclization in aqueous media.RSC Adv. 2019 Apr 30;9(23):13249-13253. doi: 10.1039/c9ra02466b. eCollection 2019 Apr 25. RSC Adv. 2019. PMID: 35520758 Free PMC article.
References
-
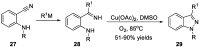
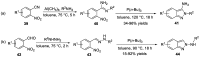
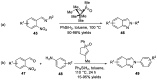
- Behrouz S. Highly efficient one-pot three component synthesis of 2H-indazoles by consecutive condensation, C-N and N-N bond formations using Cu/Aminoclay/reduced grapheme oxide nanohybrid. J. Heterocyclic. Chem. 2017;54:1863–1871. doi: 10.1002/jhet.2777. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

