Three-Dimensional Bioprinting of Articular Cartilage: A Systematic Review
- PMID: 30373384
- PMCID: PMC7755962
- DOI: 10.1177/1947603518809410
Three-Dimensional Bioprinting of Articular Cartilage: A Systematic Review
Abstract
Objective: Treatment of chondral injury is clinically challenging. Available chondral repair/regeneration techniques have significant shortcomings. A viable and durable tissue engineering strategy for articular cartilage repair remains an unmet need. Our objective was to systematically evaluate the published data on bioprinted articular cartilage with regards to scaffold-based, scaffold-free and in situ cartilage bioprinting.
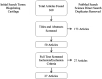
Design: We performed a systematic review of studies using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed and ScienceDirect databases were searched and all articles evaluating the use of 3-dimensional (3D) bioprinting in articular cartilage were included. Inclusion criteria included studies written in or translated to English, published in a peer-reviewed journal, and specifically discussing bioinks and/or bioprinting of living cells related to articular cartilage applications. Review papers, articles in a foreign language, and studies not involving bioprinting of living cells related to articular cartilage applications were excluded.
Results: Twenty-seven studies for articular cartilage bioprinting were identified that met inclusion and exclusion criteria. The technologies, materials, cell types used in these studies, and the biological and physical properties of the created constructs have been demonstrated.
Conclusion: These 27 studies have demonstrated 3D bioprinting of articular cartilage to be a tissue engineering strategy that has tremendous potential translational value. The unique abilities of the varied techniques allow replication of mechanical properties and advances toward zonal differentiation. This review demonstrates that bioprinting has great capacity for clinical cartilage reconstruction and future in vivo implantation.
Keywords: articular cartilage; bioprinting; scaffold-free; tissue engineering; zonal structure.
Conflict of interest statement
Figures


Similar articles
-
3D Bioprinting Strategies for Articular Cartilage Tissue Engineering.Ann Biomed Eng. 2024 Jul;52(7):1883-1893. doi: 10.1007/s10439-023-03236-8. Epub 2023 May 18. Ann Biomed Eng. 2024. PMID: 37204546 Review.
-
Application and development of 3D bioprinting in cartilage tissue engineering.Biomater Sci. 2022 Sep 27;10(19):5430-5458. doi: 10.1039/d2bm00709f. Biomater Sci. 2022. PMID: 35972308 Review.
-
Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs.Acta Biomater. 2020 Apr 1;106:114-123. doi: 10.1016/j.actbio.2020.01.046. Epub 2020 Feb 3. Acta Biomater. 2020. PMID: 32027992
-
Biomechanical issues of tissue-engineered constructs for articular cartilage regeneration: in vitro and in vivo approaches.Br Med Bull. 2019 Dec 11;132(1):53-80. doi: 10.1093/bmb/ldz034. Br Med Bull. 2019. PMID: 31854445 Review.
-
3D Bioprinting for Cartilage and Osteochondral Tissue Engineering.Adv Healthc Mater. 2017 Nov;6(22). doi: 10.1002/adhm.201700298. Epub 2017 Aug 14. Adv Healthc Mater. 2017. PMID: 28804984 Review.
Cited by
-
Additive Manufacturing Strategies for Personalized Drug Delivery Systems and Medical Devices: Fused Filament Fabrication and Semi Solid Extrusion.Molecules. 2022 Apr 27;27(9):2784. doi: 10.3390/molecules27092784. Molecules. 2022. PMID: 35566146 Free PMC article. Review.
-
Valuable effect of Manuka Honey in increasing the printability and chondrogenic potential of a naturally derived bioink.Mater Today Bio. 2022 May 13;14:100287. doi: 10.1016/j.mtbio.2022.100287. eCollection 2022 Mar. Mater Today Bio. 2022. PMID: 35647514 Free PMC article.
-
Arthroscopic device with bendable tip for the controlled extrusion of hydrogels on cartilage defects.Sci Rep. 2024 Aug 27;14(1):19904. doi: 10.1038/s41598-024-70426-2. Sci Rep. 2024. PMID: 39191817 Free PMC article.
-
Aspiration-assisted bioprinting of the osteochondral interface.Sci Rep. 2020 Aug 4;10(1):13148. doi: 10.1038/s41598-020-69960-6. Sci Rep. 2020. PMID: 32753630 Free PMC article.
-
Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage.J Biomed Mater Res B Appl Biomater. 2019 Nov;107(8):2649-2657. doi: 10.1002/jbm.b.34354. Epub 2019 Mar 12. J Biomed Mater Res B Appl Biomater. 2019. PMID: 30860678 Free PMC article.
References
-
- Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994-1009. - PubMed
-
- Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95:1045-54. - PubMed
-
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. - PubMed
-
- Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117-24. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

