Sirolimus is efficacious in treatment for extensive and/or complex slow-flow vascular malformations: a monocentric prospective phase II study
- PMID: 30373605
- PMCID: PMC6206885
- DOI: 10.1186/s13023-018-0934-z
Sirolimus is efficacious in treatment for extensive and/or complex slow-flow vascular malformations: a monocentric prospective phase II study
Abstract
Background: Extensive and complex vascular malformations often cause chronic pain and severe functional restraint. Conventional treatments, such as surgery and/or sclerotherapy, are rarely curative, underscoring the great need for new therapeutic modalities. Recent preclinical and clinical data demonstrated that sirolimus could offset the progression of vascular malformations and significantly improve quality of life of patients through inhibition of the Phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian Target of Rapamycin (mTOR) pathway. The purpose of this prospective study was to assess the efficacy and safety of this treatment in patients with extensive or complex slow-flow vascular malformations.
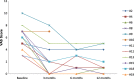
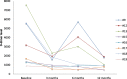
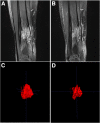
Methods: Sirolimus was administered orally on a continuous dosing schedule with pharmacokinetic-guided target serum concentration level of 10 to 15 ng/ml. Patients were seen every month for the first three months and subsequently every three months. The primary endpoints were safety and efficacy, based on clinical, biological and radiological evaluations, as well as a quality of life questionnaire.
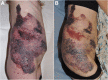
Results: Nineteen patients, from 3 to 64 years old, with lymphatic (LM), venous (VM) or complex slow-flow malformations, refractory to standard care, were enrolled and received sirolimus continuously. After 12 months of follow-up, 16 patients were available for assessment of efficacy and safety: all had a significant and rapid improvement of their symptoms and quality of life. In two patients, sirolimus treatment permitted sclerotherapy and surgery, initially evaluated unfeasible. Sirolimus was well tolerated, with mucositis as the most common (10% of patients) grade 3 adverse event.
Conclusions: Sirolimus was efficient in extensive LM, VM and/or complex malformations that were refractory to conventional treatments and was well tolerated.
Keywords: Complex vascular malformation; Extensive vascular anomaly; Lymphatic malformation; Rapamycin; Sirolimus; Slow-flow anomaly; Venous malformation.
Conflict of interest statement
Ethics approval and consent to participate
This study (NCT01811667; EudraCT 2012–001262-15) was approved by the Ethics Committee of the Cliniques universitaires Saint-Luc, Brussels, Belgium. Each patient or patient’s parent signed an informed consent after receiving a summary explaining the procedure of the study. The trial was also registered at
Consent for publication
Each patient or patient’s parent signed a consent for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Treatment of voluminous and complicated superficial slow-flow vascular malformations with sirolimus (PERFORMUS): protocol for a multicenter phase 2 trial with a randomized observational-phase design.Trials. 2018 Jun 27;19(1):340. doi: 10.1186/s13063-018-2725-1. Trials. 2018. PMID: 29945674 Free PMC article.
-
Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies.Pediatrics. 2016 Feb;137(2):e20153257. doi: 10.1542/peds.2015-3257. Epub 2016 Jan 18. Pediatrics. 2016. PMID: 26783326 Free PMC article. Clinical Trial.
-
Sirolimus (Rapamycin) for Slow-Flow Malformations in Children: The Observational-Phase Randomized Clinical PERFORMUS Trial.JAMA Dermatol. 2021 Nov 1;157(11):1289-1298. doi: 10.1001/jamadermatol.2021.3459. JAMA Dermatol. 2021. PMID: 34524406 Free PMC article. Clinical Trial.
-
A narrative review of the role of sirolimus in the treatment of congenital vascular malformations.J Vasc Surg Venous Lymphat Disord. 2021 Sep;9(5):1321-1333. doi: 10.1016/j.jvsv.2021.03.001. Epub 2021 Mar 15. J Vasc Surg Venous Lymphat Disord. 2021. PMID: 33737259 Review.
-
Treatment of Lymphatic Malformations with the mTOR Inhibitor Sirolimus: A Systematic Review.Lymphat Res Biol. 2018 Aug;16(4):330-339. doi: 10.1089/lrb.2017.0062. Epub 2018 Jun 20. Lymphat Res Biol. 2018. PMID: 29924669
Cited by
-
Cellular and molecular mechanisms of PIK3CA-related vascular anomalies.Vasc Biol. 2019 May 28;1(1):H33-H40. doi: 10.1530/VB-19-0016. eCollection 2019. Vasc Biol. 2019. PMID: 32923951 Free PMC article. Review.
-
Bockenheimer disease is associated with a TEK variant.Cold Spring Harb Mol Case Stud. 2021 Dec 9;7(6):a006119. doi: 10.1101/mcs.a006119. Print 2021 Dec. Cold Spring Harb Mol Case Stud. 2021. PMID: 34649969 Free PMC article.
-
Magnitude and relevance of change in health-related quality of life in patients with vascular malformations treated with sirolimus.Front Med (Lausanne). 2023 Apr 20;10:1155476. doi: 10.3389/fmed.2023.1155476. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37153086 Free PMC article.
-
MEK inhibition reduced vascular tumor growth and coagulopathy in a mouse model with hyperactive GNAQ.Nat Commun. 2023 Apr 6;14(1):1929. doi: 10.1038/s41467-023-37516-7. Nat Commun. 2023. PMID: 37024491 Free PMC article.
-
Effectiveness and safety of sirolimus in the treatment of venous malformations: A meta-analysis of prospective studies.J Vasc Surg Venous Lymphat Disord. 2025 Jul 5;13(6):102284. doi: 10.1016/j.jvsv.2025.102284. Online ahead of print. J Vasc Surg Venous Lymphat Disord. 2025. PMID: 40619100 Free PMC article. Review.
References
-
- Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al. ISSVA board and scientific committee. Vascular anomalies classification: recommendations from the International Society for the Study of vascular anomalies. Pediatrics. 2015;136:203–214. doi: 10.1542/peds.2014-3673. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

