Early therapeutic plasma exchange in septic shock: a prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers
- PMID: 30373638
- PMCID: PMC6206942
- DOI: 10.1186/s13054-018-2220-9
Early therapeutic plasma exchange in septic shock: a prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers
Abstract
Background: Given the pathophysiological key role of the host response to an infection rather than the infection per se, an ideal therapeutic strategy would also target this response. This study was designed to demonstrate safety and feasibility of early therapeutic plasma exchange (TPE) in severely ill individuals with septic shock.
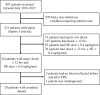
Methods: This was a prospective single center, open-label, nonrandomized pilot study enrolling 20 patients with early septic shock (onset < 12 h) requiring high doses of norepinephrine (NE; > 0.4 μg/kg/min) out of 231 screened septic patients. Clinical and biochemical data were obtained before and after TPE. Plasma samples were taken for ex-vivo stimulation of human umbilical vein endothelial cells (HUVECs) to analyze barrier function (immunocytochemistry and transendothelial electrical resistance (TER)). Cytokines were measured by cytometric bead array (CBA) and enzyme-linked immunosorbent assays (ELISAs). An immediate response was defined as > 20% NE reduction from baseline to the end of TPE.
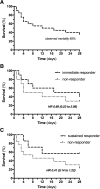
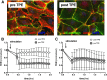
Results: TPE was well tolerated without the occurrence of any adverse events and was associated with a rapid reduction in NE (0.82 (0.61-1.17) vs. 0.56 (0.41-0.78) μg/kg/min, p = 0.002) to maintain mean arterial pressure (MAP) above 65 mmHg. The observed 28-day mortality was 65%. Key proinflammatory cytokines and permeability factors (e.g., interleukin (IL)-6, IL-1b, and angiopoietin-2) were significantly reduced after TPE, while the protective antipermeability factor angiopoietin-1 was not changed. Ex-vivo stimulation of HUVECs with plasma obtained before TPE induced substantial cellular hyperpermeability, which was completely abolished with plasma obtained after TPE.
Conclusions: Inclusion of early septic shock patients with high doses of vasopressors was feasible and TPE was safe. Rapid hemodynamic improvement and favorable changes in the cytokine profile in patients with septic shock were observed. It has yet to be determined whether early TPE also improves outcomes in this patient cohort. An appropriately powered multicenter randomized controlled trial is desirable.
Trial registration: Clinicaltrials.gov, NCT03065751 . Retrospectively registered on 28 February 2017.
Keywords: Blood purification; Endothelium; Extracorporeal treatment; Fresh frozen plasma; Plasmapheresis.
Conflict of interest statement
Ethics approval and consent to participate
The ethical committee of Hannover Medical School approved the protocol (no. 2786–2015), and written informed consent was obtained from participants or authorized representatives. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. This study was designed to prove feasibility of a planned multicenter RCT (clinicaltrials.gov NCT03065751).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

