Subclonal TP53 copy number is associated with prognosis in multiple myeloma
- PMID: 30373884
- PMCID: PMC6533595
- DOI: 10.1182/blood-2018-06-857250
Subclonal TP53 copy number is associated with prognosis in multiple myeloma
Abstract
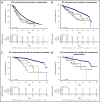
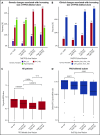
Multiple myeloma (MM) is a genetically heterogeneous cancer of bone marrow plasma cells with variable outcome. To assess the prognostic relevance of clonal heterogeneity of TP53 copy number, we profiled tumors from 1777 newly diagnosed Myeloma XI trial patients with multiplex ligation-dependent probe amplification (MLPA). Subclonal TP53 deletions were independently associated with shorter overall survival, with a hazard ratio of 1.8 (95% confidence interval, 1.2-2.8; P = .01). Clonal, but not subclonal, TP53 deletions were associated with clinical markers of advanced disease, specifically lower platelet counts (P < .001) and increased lactate dehydrogenase (P < .001), as well as a higher frequency of features indicative of genomic instability, del(13q) (P = .002) or del(1p) (P = .006). Biallelic TP53 loss-of-function by mutation and deletion was rare (2.4%) and associated with advanced disease. We present a framework for identifying subclonal TP53 deletions by MLPA, to improve patient stratification in MM and tailor therapy, enabling management strategies.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: V.S. received travel support from Sanofi and Janssen. S.S. is an employee of MRC-Holland. M.W.J. acted as a consultant for Janssen, Takeda, Amgen, Celgene Corporation, and Novartis; received travel support and honoraria from Janssen, Takeda, and Amgen; received honoraria from Celgene Corporation and Novartis; and received research funding from Janssen and Celgene Corporation. M.T.D. has equity ownership in and serves on the board of directors of Abingdon Health. R.G.O. received honoraria from Takeda and Celgene Corporation; received travel support from Takeda and Janssen; acted as a consultant for Janssen and Celgene Corporation; and received research funding from Celgene Corporation. G.J.M. received research funding from Janssen and Celgene Corporation and acted as a consultant for and received research funding from Bristol-Myers Squibb, Takeda, and Celgene Corporation. W.M.G. received research funding from Celgene Corporation, Amgen, Merck Sharp and Dohme; acted as a consultant for Celgene Corporation; received honoraria from Janssen and Abbvie. F.E.D. acted as a consultant for and received honoraria from Amgen, AbbVie, Takeda, Janssen, and Celgene Corporation. G.C. acted as a consultant for and received honoraria from Takeda, Glycomimetics, Sanofi, Celgene Corporation, Janssen, Bristol-Myers Squibb, and Amgen; received research funding from Takeda, Celgene Corporation, Janssen, and Amgen; and is a member of the speakers bureau for Sanofi, Celgene Corporation, Janssen, and Amgen. D.A.C. received research funding from Celgene Corporation, Amgen, and Merck Sharp and Dohme. G.J. acted as a consultant for and received honoraria from Roche, Amgen, Janssen, Merck Sharp and Dohme, Celgene Corporation, and Takeda; received travel support from Celgene Corporation and Takeda; received research funding from Takeda; and is a member of the speakers bureau for Roche, Amgen, Janssen, Merck Sharp and Dohme, Celgene Corporation, and Takeda. M.F.K. acted as a consultant for Bristol-Myers Squibb, Chugai, Janssen, Amgen, Takeda, and Celgene Corporation; received travel support from Bristol-Myers Squibb and Takeda; received honoraria from Janssen, Amgen, and Celgene Corporation; and received research funding from Celgene Corporation. The remaining authors declare no competing financial interests.
Figures

References
-
- McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613-628. - PubMed
-
- Manier S, Salem KZ, Park J, Landau DA, Getz G, Ghobrial IM. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2017;14(2):100-113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

