Locking loop movement in the ubiquinone pocket of complex I disengages the proton pumps
- PMID: 30374105
- PMCID: PMC6206036
- DOI: 10.1038/s41467-018-06955-y
Locking loop movement in the ubiquinone pocket of complex I disengages the proton pumps
Abstract
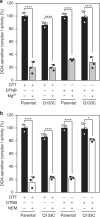
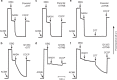
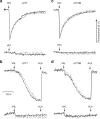
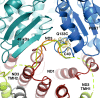
Complex I (proton-pumping NADH:ubiquinone oxidoreductase) is the largest enzyme of the mitochondrial respiratory chain and a significant source of reactive oxygen species (ROS). We hypothesized that during energy conversion by complex I, electron transfer onto ubiquinone triggers the concerted rearrangement of three protein loops of subunits ND1, ND3, and 49-kDa thereby generating the power-stoke driving proton pumping. Here we show that fixing loop TMH1-2ND3 to the nearby subunit PSST via a disulfide bridge introduced by site-directed mutagenesis reversibly disengages proton pumping without impairing ubiquinone reduction, inhibitor binding or the Active/Deactive transition. The X-ray structure of mutant complex I indicates that the disulfide bridge immobilizes but does not displace the tip of loop TMH1-2ND3. We conclude that movement of loop TMH1-2ND3 located at the ubiquinone-binding pocket is required to drive proton pumping corroborating one of the central predictions of our model for the mechanism of energy conversion by complex I proposed earlier.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Structure and function of mitochondrial complex I.Biochim Biophys Acta. 2016 Jul;1857(7):902-14. doi: 10.1016/j.bbabio.2016.02.013. Epub 2016 Feb 24. Biochim Biophys Acta. 2016. PMID: 26921811 Review.
-
Essential role of accessory subunit LYRM6 in the mechanism of mitochondrial complex I.Nat Commun. 2020 Nov 26;11(1):6008. doi: 10.1038/s41467-020-19778-7. Nat Commun. 2020. PMID: 33243981 Free PMC article.
-
Structural biology. Mechanistic insight from the crystal structure of mitochondrial complex I.Science. 2015 Jan 2;347(6217):44-9. doi: 10.1126/science.1259859. Science. 2015. PMID: 25554780
-
Complex I function in mitochondrial supercomplexes.Biochim Biophys Acta. 2016 Jul;1857(7):991-1000. doi: 10.1016/j.bbabio.2016.01.013. Epub 2016 Jan 25. Biochim Biophys Acta. 2016. PMID: 26820434 Review.
-
Tracing the tail of ubiquinone in mitochondrial complex I.Biochim Biophys Acta. 2012 Oct;1817(10):1776-84. doi: 10.1016/j.bbabio.2012.03.021. Epub 2012 Mar 29. Biochim Biophys Acta. 2012. PMID: 22484275
Cited by
-
Ubiquinone Binding and Reduction by Complex I-Open Questions and Mechanistic Implications.Front Chem. 2021 Apr 30;9:672851. doi: 10.3389/fchem.2021.672851. eCollection 2021. Front Chem. 2021. PMID: 33996767 Free PMC article. Review.
-
Role of Second Quinone Binding Site in Proton Pumping by Respiratory Complex I.Front Chem. 2019 Apr 9;7:221. doi: 10.3389/fchem.2019.00221. eCollection 2019. Front Chem. 2019. PMID: 31024903 Free PMC article.
-
Deactivation blocks proton pathways in the mitochondrial complex I.Proc Natl Acad Sci U S A. 2021 Jul 20;118(29):e2019498118. doi: 10.1073/pnas.2019498118. Proc Natl Acad Sci U S A. 2021. PMID: 34272275 Free PMC article.
-
Quinone chemistry in respiratory complex I involves protonation of a conserved aspartic acid residue.FEBS Lett. 2024 Dec;598(23):2856-2865. doi: 10.1002/1873-3468.15013. Epub 2024 Sep 11. FEBS Lett. 2024. PMID: 39262040 Free PMC article.
-
Structural basis for a complex I mutation that blocks pathological ROS production.Nat Commun. 2021 Jan 29;12(1):707. doi: 10.1038/s41467-021-20942-w. Nat Commun. 2021. PMID: 33514727 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- ZI552/4-1/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- EXC115 CEF/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- CRC746/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- BR1633/3-1/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- EXC 115 CEF/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
LinkOut - more resources
Full Text Sources

