Respiratory syncytial virus hospitalization and incurred morbidities the season after prophylaxis
- PMID: 30374219
- PMCID: PMC6199632
- DOI: 10.1093/pch/pxy046
Respiratory syncytial virus hospitalization and incurred morbidities the season after prophylaxis
Abstract
Objectives: The primary objective of this study was to determine the incidence and incurred morbidities of Respiratory syncytial virus (RSV)-related hospitalization (RSVH), the season following completion of prophylaxis.
Methods: A retrospective study was conducted of all infants enrolled in a prophylaxis clinic in one institution during the 2009 to 2014 RSV seasons. RSV infection was identified by Diseases codes and confirmed by RSV-positivity. Data were classified into five groups based on indications for prophylaxis. The incidence of RSVH was calculated. For each subgroup, differences in characteristics between children with and without RSVH were analyzed by independent t test or chi-square test.
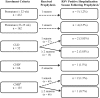
Results: During five RSV seasons, 827 infants were enrolled. RSVH incidence the season following prophylaxis was 2.1% (n=17/827). Children with chronic lung disease (CLD) had the highest RSVH incidence (7.7%; n=4/52) followed by preterms 33 to 35 weeks gestation (2.5%; n=4/162), those with complex medical disorders (2.2%; n=3/135), those with congenital heart disease (1.5%; n=1/66) and preterms less than or equal to 32 weeks gestation (1.2%; n=5/412). There was no statistically significant association between indications for prophylaxis and RSVH (Fisher exact test, P=0.060). The odds of RSVH were 4.9 times greater (odds ratio [OR]=4.9; 95% CI: 1.53, 15.55; P=0.007) in CLD compared to those without CLD. The median length of RSVH stay was 4 days; 58.8% (n=10/17) required oxygen (median 1 day); 29.4% (n=5/17) required intensive care.
Conclusions: Infants with CLD are at highest risk for RSVH in the season postprophylaxis and may merit palivizumab for more than two seasons dependent on disease severity. However, larger prospective studies are necessary to confirm the findings before embarking on a strategy of providing prophylaxis for a third RSV season.
Keywords: Hospitalization; Outcomes; Palivizumab; Respiratory syncytial virus infection; Season after immunization.
Figures
Similar articles
-
First versus second year respiratory syncytial virus prophylaxis in chronic lung disease (2005-2015).Eur J Pediatr. 2017 Mar;176(3):413-422. doi: 10.1007/s00431-017-2849-4. Epub 2017 Jan 20. Eur J Pediatr. 2017. PMID: 28105526 Free PMC article.
-
Respiratory syncytial virus prophylaxis in infants with congenital airway anomalies compared to standard indications and complex medical disorders.Eur J Pediatr. 2019 Mar;178(3):377-385. doi: 10.1007/s00431-018-03308-1. Epub 2019 Jan 4. Eur J Pediatr. 2019. PMID: 30610419
-
Respiratory illness and respiratory syncytial virus hospitalization in infants with a tracheostomy following prophylaxis with palivizumab.Eur J Clin Microbiol Infect Dis. 2019 Aug;38(8):1561-1568. doi: 10.1007/s10096-019-03588-x. Epub 2019 May 22. Eur J Clin Microbiol Infect Dis. 2019. PMID: 31119575
-
Rationale for full-season dosing for passive antibody prophylaxis of respiratory syncytial virus.Hum Vaccin Immunother. 2014;10(3):607-14. doi: 10.4161/hv.27426. Epub 2013 Dec 6. Hum Vaccin Immunother. 2014. PMID: 24316863 Free PMC article. Review.
-
Defining the Risk and Associated Morbidity and Mortality of Severe Respiratory Syncytial Virus Infection Among Infants with Chronic Lung Disease.Infect Dis Ther. 2016 Dec;5(4):453-471. doi: 10.1007/s40121-016-0137-7. Epub 2016 Nov 18. Infect Dis Ther. 2016. PMID: 27864751 Free PMC article. Review.
Cited by
-
Health economic evaluation of implementing a universal immunization program with nirsevimab compared to standard of care for the prevention of respiratory syncytial virus disease in Canadian infants.Hum Vaccin Immunother. 2025 Dec;21(1):2480875. doi: 10.1080/21645515.2025.2480875. Epub 2025 Apr 5. Hum Vaccin Immunother. 2025. PMID: 40186452 Free PMC article.
-
Number needed to immunize to prevent RSV with extended half-life monoclonal antibody.Vaccine. 2020 Jul 22;38(34):5474-5479. doi: 10.1016/j.vaccine.2020.06.034. Epub 2020 Jun 26. Vaccine. 2020. PMID: 32600912 Free PMC article.
References
LinkOut - more resources
Full Text Sources
