Role of TNF-α in Regulating the Exercise Pressor Reflex in Rats With Femoral Artery Occlusion
- PMID: 30374312
- PMCID: PMC6196241
- DOI: 10.3389/fphys.2018.01461
Role of TNF-α in Regulating the Exercise Pressor Reflex in Rats With Femoral Artery Occlusion
Abstract
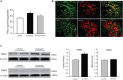
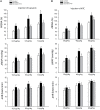
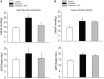
Responses of sympathetic nerve activity and arterial blood pressure are augmented during activation of the exercise pressor reflex in rats with femoral artery occlusion. The present study examined the role played by proinflammatory tumor necrosis factor-α (TNF-α) in regulating augmented sympathetic responsiveness induced by stimulation of muscle metabolic receptors and static muscle contraction following 72 h of femoral artery occlusion. We first observed that the levels of TNF-α and protein expression of TNF-α receptor type 1 (TNFR1) were increased in the dorsal root ganglion (DRG) of hindlimbs with femoral artery occlusion. Note that TNF-α was observed within DRG neurons of C-fiber afferent nerves. Capsaicin (TRPV1 agonist) and AITC (TRPA1 agonist) were injected into arterial blood supply of the hindlimbs to stimulate metabolically sensitive thin-fiber muscle afferents. The effects of these injections on the sympathetic and pressor responses were further examined in control rats and rats with femoral artery occlusion. As TNF-α synthesis suppressor pentoxifylline (PTX) was previously administered into the hindlimb with femoral artery occlusion, sympathetic, and pressor responses induced by capsaicin and AITC were attenuated. In occluded rats, PTX also attenuated the exaggeration of blood pressure response induced by muscle contraction, but not by passive tendon stretch. Overall, the results suggest that TNF-α plays a role in modulating exaggerated sympathetic nervous activity via the metabolic component of the exercise pressor reflex when the hindlimb muscles are ischemic in peripheral arterial disease.
Keywords: TNF-α; blood pressure; exercise; hindlimb ischemia; muscle afferent nerve; peripheral arterial disease.
Figures
Similar articles
-
Sympathetic Nerve Activity and Blood Pressure Response to Exercise in Peripheral Artery Disease: From Molecular Mechanisms, Human Studies, to Intervention Strategy Development.Int J Mol Sci. 2022 Sep 13;23(18):10622. doi: 10.3390/ijms231810622. Int J Mol Sci. 2022. PMID: 36142521 Free PMC article. Review.
-
Role for NGF in augmented sympathetic nerve response to activation of mechanically and metabolically sensitive muscle afferents in rats with femoral artery occlusion.J Appl Physiol (1985). 2012 Oct 15;113(8):1311-22. doi: 10.1152/japplphysiol.00617.2012. Epub 2012 Jun 28. J Appl Physiol (1985). 2012. PMID: 22744968 Free PMC article.
-
Proteinase-Activated Receptor-2 Sensitivity of Amplified TRPA1 Activity in Skeletal Muscle Afferent Nerves and Exercise Pressor Reflex in Rats with Femoral Artery Occlusion.Cell Physiol Biochem. 2017;44(1):163-171. doi: 10.1159/000484624. Epub 2017 Nov 6. Cell Physiol Biochem. 2017. PMID: 29131007 Free PMC article.
-
Contribution of nerve growth factor to upregulation of P2X₃ expression in DRG neurons of rats with femoral artery occlusion.Am J Physiol Heart Circ Physiol. 2011 Sep;301(3):H1070-9. doi: 10.1152/ajpheart.00188.2011. Epub 2011 Jun 3. Am J Physiol Heart Circ Physiol. 2011. PMID: 21642505 Free PMC article.
-
Peripheral Mechanisms of Ischemic Myalgia.Front Cell Neurosci. 2017 Dec 22;11:419. doi: 10.3389/fncel.2017.00419. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29311839 Free PMC article. Review.
Cited by
-
Impaired hemodynamic response to exercise in patients with peripheral artery disease: evidence of a link to inflammation and oxidative stress.Am J Physiol Regul Integr Comp Physiol. 2022 Nov 1;323(5):R710-R719. doi: 10.1152/ajpregu.00159.2022. Epub 2022 Sep 26. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 36154490 Free PMC article.
-
Exaggerated exercise pressor reflex in male UC Davis type 2 diabetic rats is due to the pathophysiology of the disease and not aging.Front Physiol. 2023 Jan 10;13:1063326. doi: 10.3389/fphys.2022.1063326. eCollection 2022. Front Physiol. 2023. PMID: 36703927 Free PMC article.
-
Sympathetic Nerve Activity and Blood Pressure Response to Exercise in Peripheral Artery Disease: From Molecular Mechanisms, Human Studies, to Intervention Strategy Development.Int J Mol Sci. 2022 Sep 13;23(18):10622. doi: 10.3390/ijms231810622. Int J Mol Sci. 2022. PMID: 36142521 Free PMC article. Review.
-
Enhancement by TNF-α of TTX-resistant NaV current in muscle sensory neurons after femoral artery occlusion.Am J Physiol Regul Integr Comp Physiol. 2020 Apr 1;318(4):R772-R780. doi: 10.1152/ajpregu.00338.2019. Epub 2020 Feb 26. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32101460 Free PMC article.
-
One-Time Acute Heat Treatment Is Effective for Attenuation of the Exaggerated Exercise Pressor Reflex in Rats With Femoral Artery Occlusion.Front Physiol. 2020 Aug 6;11:942. doi: 10.3389/fphys.2020.00942. eCollection 2020. Front Physiol. 2020. PMID: 32848871 Free PMC article.
References
-
- Bessou P., Laporte Y. (1958). Activation of non-medullated afferent fibers of muscular origin. C. R. Seances Soc. Biol. Fil. 152 1587–1590. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

