Conjunctival Inflammatory Gene Expression Profiling in Dry Eye Disease: Correlations With HLA-DRA and HLA-DRB1
- PMID: 30374345
- PMCID: PMC6196257
- DOI: 10.3389/fimmu.2018.02271
Conjunctival Inflammatory Gene Expression Profiling in Dry Eye Disease: Correlations With HLA-DRA and HLA-DRB1
Abstract
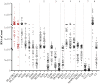
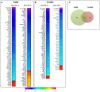
Purpose: In several multicenter clinical trials, HLA-DR was found to be a potential biomarker of dry eye disease (DED)'s severity and prognosis. Given the fact that HLA-DR receptor is a heterodimer consisting in an alpha and a beta chain, we intended to investigate the correlation of inflammatory targets with the corresponding transcripts, HLA-DRA and HLA-DRB1, to characterize specific targets closely related to HLA-DR expressed in conjunctival cells from patients suffering from DED of various etiologies. Methods: A prospective study was conducted in 88 patients with different forms of DED. Ocular symptom scores, ocular-staining grades, tear breakup time (TBUT) and Schirmer test were evaluated. Superficial conjunctival cells were collected by impression cytology and total RNAs were extracted for analyses using the new NanoString® nCounter technology based on an inflammatory human code set containing 249 inflammatory genes. Results: Two hundred transcripts were reliably detected in conjunctival specimens at various levels ranging from 1 to 222,546 RNA copies. Overall, from the 88 samples, 21 target genes showed a highly significant correlation (R > 0.8) with HLA-DRA and HLA-DRB1, HLA-DRA and B1 presenting the highest correlation (R = 0.9). These selected targets belonged to eight family groups, namely interferon and interferon-stimulated genes, tumor necrosis factor superfamily and related factors, Toll-like receptors and related factors, complement system factors, chemokines/cytokines, the RIPK enzyme family, and transduction signals such as the STAT and MAPK families. Conclusions: We have identified a profile of 21 transcripts correlated with HLA-DR expression, suggesting closely regulated signaling pathways and possible direct or indirect interactions between them. The NanoString® nCounter technology in conjunctival imprints could constitute a reliable tool in the future for wider screening of inflammatory biomarkers in DED, usable in very small samples. Broader combinations of biomarkers associated with HLA-DR could be analyzed to develop new diagnostic approaches, identify tighter pathophysiological gene signatures and personalize DED therapies more efficiently.
Keywords: HLA-DR; NanoString® assay; conjunctival imprints; dry eye disease; inflammatory targets.
Figures

Similar articles
-
Correlation of clinical symptoms and signs with conjunctival gene expression in primary Sjögren syndrome dry eye patients.Ocul Surf. 2019 Jul;17(3):516-525. doi: 10.1016/j.jtos.2019.03.005. Epub 2019 Mar 21. Ocul Surf. 2019. PMID: 30905840
-
Correlation Between the Inflammatory Marker HLA-DR and Signs and Symptoms in Moderate to Severe Dry Eye Disease.Invest Ophthalmol Vis Sci. 2017 Apr 1;58(4):2438-2448. doi: 10.1167/iovs.15-16555. Invest Ophthalmol Vis Sci. 2017. PMID: 28448672
-
Association of Conjunctival Cell HLA-DR Expression with the Severity of Signs and Symptoms of Dry Eye Disease at Baseline in the NORTHERN LIGHTS Phase 2 Trial.Curr Eye Res. 2025 Jun;50(6):567-571. doi: 10.1080/02713683.2025.2469254. Epub 2025 Mar 7. Curr Eye Res. 2025. PMID: 40052360 Clinical Trial.
-
Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting.Acta Ophthalmol. 2018 Mar;96(2):111-119. doi: 10.1111/aos.13436. Epub 2017 Apr 8. Acta Ophthalmol. 2018. PMID: 28390092 Free PMC article. Review.
-
Molecular and cellular biomarkers in dry eye disease and ocular allergy.Curr Opin Allergy Clin Immunol. 2012 Oct;12(5):523-33. doi: 10.1097/ACI.0b013e328357b488. Curr Opin Allergy Clin Immunol. 2012. PMID: 22895048 Review.
Cited by
-
A Distinguishable Peripheral Blood and Conjunctival Transcriptome and Gut Microbiome in Sjögren's Disease: A Pilot Study.Eye Contact Lens. 2025 May 2;51(7):304-311. doi: 10.1097/ICL.0000000000001186. Eye Contact Lens. 2025. PMID: 40314468 Free PMC article.
-
Update on the role of impression cytology in ocular surface disease.Taiwan J Ophthalmol. 2019 Sep 12;9(3):141-149. doi: 10.4103/tjo.tjo_57_19. eCollection 2019 Jul-Sep. Taiwan J Ophthalmol. 2019. PMID: 31572650 Free PMC article. Review.
-
Comparison of Two Experimental Mouse Dry Eye Models through Inflammatory Gene Set Enrichment Analysis Based on a Multiplexed Transcriptomic Approach.Int J Mol Sci. 2021 Oct 5;22(19):10770. doi: 10.3390/ijms221910770. Int J Mol Sci. 2021. PMID: 34639111 Free PMC article.
-
NLRP3 Inflammasome as a Potential Therapeutic Target in Dry Eye Disease.Int J Mol Sci. 2023 Jun 29;24(13):10866. doi: 10.3390/ijms241310866. Int J Mol Sci. 2023. PMID: 37446038 Free PMC article. Review.
-
Using Network Pharmacology and Molecular Docking to Explore the Mechanism of Qiju Dihuang Pill against Dry Eye Disease.Comput Math Methods Med. 2022 Dec 22;2022:7316794. doi: 10.1155/2022/7316794. eCollection 2022. Comput Math Methods Med. 2022. PMID: 36590763 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

