Iron Chelation with Transdermal Deferoxamine Accelerates Healing of Murine Sickle Cell Ulcers
- PMID: 30374417
- PMCID: PMC6203233
- DOI: 10.1089/wound.2018.0789
Iron Chelation with Transdermal Deferoxamine Accelerates Healing of Murine Sickle Cell Ulcers
Abstract
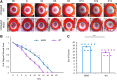
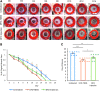
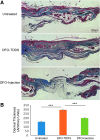
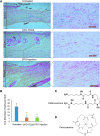
Objective: Sickle cell ulcers (SCUs) are a devastating comorbidity affecting patients with sickle cell disease (SCD). SCUs form over the medial or lateral malleoli of the lower extremity, are slow to heal, and prone to recidivism. Some SCUs may never heal, leading to chronic pain and foot deformities. There is no specific and effective therapy for SCUs. Systemic deferoxamine (DFO) has been demonstrated to prevent some of the sequelae of SCD by chelating iron. In this study, we tested the ability of DFO delivered via a transdermal delivery system (DFO-TDDS) to accelerate healing in a murine model of SCU. Approach: Excisional wounds were created in a transgenic murine model of SCD expressing >99% human sickle hemoglobin, and healing rates were compared with wounds in wild-type mice. Next, excisional wounds in SCD mice were treated with DFO-TDDS, DFO injection, or left untreated. Wound closure rates, histology, and iron in the healed wounds were analyzed. Results: Wounds in SCD mice healed significantly slower than wild-type mice (***p < 0.001). DFO-TDDS-treated wounds demonstrated significantly accelerated time to closure, reduced size, and improved wound remodeling compared with untreated wounds (***p < 0.001) and DFO injection treatment (*p < 0.05). DFO released from the TDDS into wounds resulted in chelation of excessive dermal-free iron. Innovation: DFO-TDDS is a novel therapeutic that is effective in healing wounds in sickle cell mice. Conclusion: DFO-TDDS significantly accelerates healing of murine SCUs by chelation of excessive free iron and is currently manufactured in an FDA-compliant facility to be translated for treating human SCUs.
Keywords: deferoxamine; iron chelator; sickle cell disease; sickle cell ulcers; wound healing.
Figures

Similar articles
-
Transdermal deferoxamine administration improves excisional wound healing in chronically irradiated murine skin.J Transl Med. 2022 Jun 17;20(1):274. doi: 10.1186/s12967-022-03479-4. J Transl Med. 2022. PMID: 35715816 Free PMC article.
-
Optimization of transdermal deferoxamine leads to enhanced efficacy in healing skin wounds.J Control Release. 2019 Aug 28;308:232-239. doi: 10.1016/j.jconrel.2019.07.009. Epub 2019 Jul 9. J Control Release. 2019. PMID: 31299261
-
Transdermal deferoxamine prevents pressure-induced diabetic ulcers.Proc Natl Acad Sci U S A. 2015 Jan 6;112(1):94-9. doi: 10.1073/pnas.1413445112. Epub 2014 Dec 22. Proc Natl Acad Sci U S A. 2015. PMID: 25535360 Free PMC article.
-
Challenges and Opportunities of Deferoxamine Delivery for Treatment of Alzheimer's Disease, Parkinson's Disease, and Intracerebral Hemorrhage.Mol Pharm. 2021 Feb 1;18(2):593-609. doi: 10.1021/acs.molpharmaceut.0c00474. Epub 2020 Oct 9. Mol Pharm. 2021. PMID: 32926630 Free PMC article. Review.
-
Advances in Hypoxia-Inducible Factor-1α Stabilizer Deferoxamine in Tissue Engineering.Tissue Eng Part B Rev. 2023 Aug;29(4):347-357. doi: 10.1089/ten.TEB.2022.0168. Epub 2023 Feb 1. Tissue Eng Part B Rev. 2023. PMID: 36475887 Review.
Cited by
-
Deferoxamine Intradermal Delivery Patch for Treatment of a Radiation Therapy Associated Breast Wound.Ann Case Rep. 2024;9(3):1844. doi: 10.29011/2574-7754.101844. Epub 2024 Jun 13. Ann Case Rep. 2024. PMID: 39885938 Free PMC article.
-
Application of Deferoxamine in Tissue Regeneration Attributed to Promoted Angiogenesis.Molecules. 2024 Apr 29;29(9):2050. doi: 10.3390/molecules29092050. Molecules. 2024. PMID: 38731540 Free PMC article. Review.
-
Deferoxamine topical cream superior to patch in rescuing radiation-induced fibrosis of unwounded and wounded skin.J Cell Mol Med. 2024 Apr;28(8):e18306. doi: 10.1111/jcmm.18306. J Cell Mol Med. 2024. PMID: 38613357 Free PMC article.
-
Topical Iron Chelator Therapy: Current Status and Future Prospects.Cureus. 2023 Oct 26;15(10):e47720. doi: 10.7759/cureus.47720. eCollection 2023 Oct. Cureus. 2023. PMID: 38022031 Free PMC article. Review.
-
Deferoxamine to Minimize Fibrosis During Radiation Therapy.Adv Wound Care (New Rochelle). 2022 Oct;11(10):548-559. doi: 10.1089/wound.2021.0021. Epub 2021 Jul 26. Adv Wound Care (New Rochelle). 2022. PMID: 34074152 Free PMC article.
References
-
- Penne JR, Goodman BM, Chen IA. Sickle cell disease & wound care: Lower extremity ulcers in “crisis.” Todays Wound Clinic 2015;9:25–27
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–2031 - PubMed
-
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997;337:762–769 - PubMed
-
- Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med 1980;302:992–995 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
