Activity of Thioallyl Compounds From Garlic Against Giardia duodenalis Trophozoites and in Experimental Giardiasis
- PMID: 30374433
- PMCID: PMC6196658
- DOI: 10.3389/fcimb.2018.00353
Activity of Thioallyl Compounds From Garlic Against Giardia duodenalis Trophozoites and in Experimental Giardiasis
Abstract
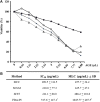
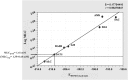
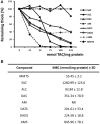
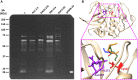
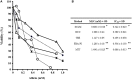
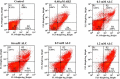
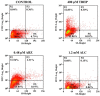
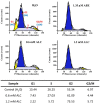
Fresh aqueous extracts (AGEs) and several thioallyl compounds (TACs) from garlic have an important antimicrobial activity that likely involves their interaction with exposed thiol groups at single aminoacids or target proteins. Since these groups are present in Giardia duodenalis trophozoites, in this work we evaluated the anti-giardial activity of AGE and several garlic's TACs. In vitro susceptibility assays showed that AGE affected trophozoite viability initially by a mechanism impairing cell integrity and oxidoreductase activities while diesterase activities were abrogated at higher AGE concentrations. The giardicidal activities of seven TACs were related to the molecular descriptor HOMO (Highest Occupied Molecular Orbital) energy and with their capacity to modify the -SH groups exposed in giardial proteins. Interestingly, the activity of several cysteine proteases in trophozoite lysates was inhibited by representative TACs as well as the cytopathic effect of the virulence factor giardipain-1. Of these, allicin showed the highest anti-giardial activity, the lower HOMO value, the highest thiol-modifying activity and the greatest inhibition of cysteine proteases. Allicin had a cytolytic mechanism in trophozoites with subsequent impairment of diesterase and oxidoreductase activities in a similar way to AGE. In addition, by electron microscopy a marked destruction of plasma membrane and endomembranes was observed in allicin-treated trophozoites while cytoskeletal elements were not affected. In further flow cytometry analyses pro-apoptotic effects of allicin concomitant to partial cell cycle arrest at G2 phase with the absence of oxidative stress were observed. In experimental infections of gerbils, the intragastric administration of AGE or allicin decreased parasite numbers and eliminated trophozoites in experimentally infected animals, respectively. These data suggest a potential use of TACs from garlic against G. duodenalis and in the treatment of giardiasis along with their additional benefits in the host's health.
Keywords: Giardia duodenalis; allicin; garlic; giardiasis; thioallyl compounds; trophozoite damage.
Figures


Similar articles
-
Giardia duodenalis Virulence - "To Be, or Not To Be".Curr Trop Med Rep. 2021;8(4):246-256. doi: 10.1007/s40475-021-00248-z. Epub 2021 Oct 21. Curr Trop Med Rep. 2021. PMID: 34697581 Free PMC article. Review.
-
Efficacy of Ageratum conyzoides extracts against Giardia duodenalis trophozoites: an experimental study.BMC Complement Med Ther. 2020 Feb 28;20(1):63. doi: 10.1186/s12906-020-2860-6. BMC Complement Med Ther. 2020. PMID: 32111225 Free PMC article.
-
Licochalcone a: A promising antiparasitic drug against giardiasis.Int J Parasitol Drugs Drug Resist. 2025 Apr;27:100573. doi: 10.1016/j.ijpddr.2024.100573. Epub 2024 Dec 12. Int J Parasitol Drugs Drug Resist. 2025. PMID: 39693890 Free PMC article.
-
Aminoguanidines: New leads for treatment of Giardia duodenalis infection.Int J Parasitol Drugs Drug Resist. 2019 Aug;10:38-44. doi: 10.1016/j.ijpddr.2019.04.003. Epub 2019 Apr 8. Int J Parasitol Drugs Drug Resist. 2019. PMID: 31015151 Free PMC article.
-
The proteasome of the differently-diverged eukaryote Giardia lamblia and its role in remodeling of the microtubule-based cytoskeleton.Crit Rev Microbiol. 2017 Aug;43(4):481-492. doi: 10.1080/1040841X.2016.1262814. Epub 2016 Dec 30. Crit Rev Microbiol. 2017. PMID: 28033730 Review.
Cited by
-
Assessment of therapeutic potential of Allium sativum and Zingiber officinale commercial supplements in experimental giardiasis models.J Parasit Dis. 2022 Sep;46(3):704-713. doi: 10.1007/s12639-022-01489-z. Epub 2022 Apr 25. J Parasit Dis. 2022. PMID: 36091266 Free PMC article.
-
Giardia duodenalis Virulence - "To Be, or Not To Be".Curr Trop Med Rep. 2021;8(4):246-256. doi: 10.1007/s40475-021-00248-z. Epub 2021 Oct 21. Curr Trop Med Rep. 2021. PMID: 34697581 Free PMC article. Review.
-
Amino acid-derived defense metabolites from plants: A potential source to facilitate novel antimicrobial development.J Biol Chem. 2021 Jan-Jun;296:100438. doi: 10.1016/j.jbc.2021.100438. Epub 2021 Feb 19. J Biol Chem. 2021. PMID: 33610552 Free PMC article. Review.
-
Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery.Chem Rev. 2021 Mar 24;121(6):3495-3560. doi: 10.1021/acs.chemrev.0c00922. Epub 2020 Nov 9. Chem Rev. 2021. PMID: 33164487 Free PMC article. Review.
-
Garlic extract in prosthesis-related infections: a literature review.J Int Med Res. 2020 Apr;48(4):300060520913778. doi: 10.1177/0300060520913778. J Int Med Res. 2020. PMID: 32290752 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

