The Role of Inhalation Delivery Devices in COPD: Perspectives of Patients and Health Care Providers
- PMID: 30374449
- PMCID: PMC6190525
- DOI: 10.15326/jcopdf.5.2.2017.0168
The Role of Inhalation Delivery Devices in COPD: Perspectives of Patients and Health Care Providers
Abstract
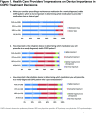
Background: Inhaled medications form the foundation of pharmacologic treatment for chronic obstructive pulmonary disease (COPD).The Delivery Makes a Difference (DMaD) project was conducted to better understand health care provider (HCP) and patient perspectives about the role of inhalation delivery devices in COPD, and to examine the nature of educational efforts between HCPs and patients on proper device technique. Methods: Data were derived from 2 original quantitative, web-based, descriptive, cross-sectional surveys distributed to HCPs who manage COPD (n=513) and patients with COPD (n=499) in the United States. Descriptive statistics were used to assess data across important demographic variables. Inferential statistics were used to assess differences in attitudinal, descriptive, and behavioral measures that were cross-tabulated with demographic data. Results: When prescribing medication for newly diagnosed patients with stable or unstable COPD, only 37% of HCPs considered type of device to be highly important, with only 45% of HCPs assessing device technique in every newly diagnosed patient. Patients with COPD were also relatively unconcerned with proper device technique (64% never concerned), regardless of their COPD severity. Although patients did not identify education as a significant impediment to proper device use, they reported inconsistent educational experiences. Conclusions: We found that HCPs and patients prioritize medication over device when selecting treatments, showing limited concerns about proper device use. These results highlight the need to coordinate professional education with patient-directed educational efforts to further promote proper device selection and use in COPD management.
Keywords: copd; inhalation device; inhalation therapy.
Conflict of interest statement
Sidney Braman has received honoraria for serving on advisory boards or has served as a consultant for AstraZeneca, Sunovion, and Genentech. Sandra G. Adams has been an investigator or received grants for research and continuing education from the National Institutes of Health, Veterans Affairs, The University of Texas System (Patient Safety Education Grant), the CHEST Foundation (GlaxoSmithKline Distinguished Scholar Award), AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Novartis, and Sunovion. Ruth Adewuya is an employee of the American College of Chest Physicians. Arzu Ari has received honoraria for serving on advisory boards or as a consultant for Bayer and Aerogen. She has received research support from ARC Medical. JoAnn Brooks has served on an advisory board for Eli Lilly and has participated in speakers’ bureaus for Janssen and Sage. Nicola A. Hanania has received honoraria for serving on advisory boards or as a consultant for Boehringer Ingelheim, GlaxoSmithKline, Roche, AstraZeneca, Novartis, Sanofi/Regeneron, and Teva. His institution has received research grant support from Sunovion, Mylan, Boehringer Ingelheim, Cheisi, AstraZeneca, Roche, and GlaxoSmithKline. Donald A. Mahler has served on advisory boards or speakers’ bureaus for AstraZeneca, Boehringer Ingelheim, Sunovion, Theravance, and Grifols. He has received royalties from Hillcrest Media, CRC Press, and Mapi Research Institute. Jill A. Ohar has received honoraria for serving on advisory boards for AstraZeneca, Novartis, Sunovion, and Mylan. Jay Peters has received research support from the Willard Foundation and the National Institute of Allergy and Infectious Disease. Shahin Sanjar is an employee of Sunovion.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Lung Disease (2017 Report). GOLD website. http://goldcopd.org/ Published 2017 Accessed December 2017.
-
- Dolovich MB,Ahrens RC,Hess DR,et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335-371. doi: https://doi.org/10.1378/chest.127.1.335 - PubMed
-
- Broeders ME,Sanchis J,Levy ML,Crompton GK,Dekhuijzen PNR. The ADMIT series: Issues in inhalation therapy—2. Improving technique and clinical effectiveness. Prim Care Respir J. 2009;18(2):76-82. doi: https://doi.org/10.4104/pcrj.2009.00025 - PMC - PubMed
-
- Hodder R,Price D. Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat Soft Mist inhaler. Int J Chron Obstruct Pulmon Dis. 2009;4:381-390. doi: https://doi.org/10.2147/COPD.S3391 - PMC - PubMed
-
- Yawn B,Colice GL,Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2012;7:495-502. doi: https://doi.org/10.2147/COPD.S32674 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous