Clinical experience with misoprostol vaginal insert for induction of labor: a prospective clinical observational study
- PMID: 30374645
- PMCID: PMC6328513
- DOI: 10.1007/s00404-018-4942-y
Clinical experience with misoprostol vaginal insert for induction of labor: a prospective clinical observational study
Abstract
Purpose: To provide real-world evidence using misoprostol vaginal insert (MVI) for induction of labor in nulliparous and parous women at two German Level I Centers in a prospective observational study.
Methods: Between 1 August 2014 and 1 October 2015, eligible pregnant women (≥ 36 + 0 weeks of gestation) requiring labor induction were treated with MVI. Endpoints included time to and mode of delivery rates of tocolysis use, tachysystole, uterine hypertonus or uterine hyperstimulation syndrome and newborn outcomes.
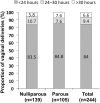
Results: Of the 354 women enrolled, 68.9% (244/354) achieved vaginal delivery (nulliparous, 139/232 [59.9%]; parous 105/122 [86.1%]; p < 0.001). Median time from MVI administration to vaginal delivery was 14.0 h (nulliparous, 14.5 h; parous, 11.9 h; p < 0.001). A total of 205/244 (84.0%) and 228/244 (93.4%) women achieved a vaginal delivery within 24 h and 30 h, respectively. The most common indications for cesarean delivery were pathologic cardiotocography (nulliparous, 41/232 [17.4%]; parous, 13/122 [10.7%]; p = 0.081) and arrested labor (dilation or descent; nulliparous, 45/232 [19.4%], parous, 3/122 [2.5%]; p ≤ 0.001). A total of 24.3% of women experienced uterine tachysystole and 9.6% experienced uterine tachysystole with fetal heart rate involvement, neither of which were significantly different for nulliparous and parous women. In total, 42/345 (12.2%) of the neonates had an arterial pH < 7.15 and 12/345 3.5% had a 5-min Apgar score ≤ 7.
Conclusion: When clinically indicated, MVI was efficient and safe for induction of labor in women with an unfavorable cervix. Women, however, should be counseled regarding the risk of uterine tachysystole prior to labor induction with MVI.
Keywords: Induction of labor; Misoprostol; Misoprostol vaginal insert; Mode of delivery; Prostaglandin E1; Time to delivery; Vaginal delivery.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees (Ethics Committee of the Department of Medicine of the Philipps-University Marburg and Ethics Committee of the Department of Medicine for the University of Duisburg-Essen: #15-6545-BO, approved August 27, 2015) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Similar articles
-
Comparative efficacy and safety of vaginal misoprostol versus dinoprostone vaginal insert in labor induction at term: a randomized trial.Arch Gynecol Obstet. 2009 Jul;280(1):19-24. doi: 10.1007/s00404-008-0843-9. Epub 2008 Nov 26. Arch Gynecol Obstet. 2009. PMID: 19034471 Clinical Trial.
-
Slow-release vaginal insert of misoprostol versus orally administrated solution of misoprostol for the induction of labour in primiparous term pregnant women: a randomised controlled trial.BJOG. 2019 Aug;126(9):1148-1155. doi: 10.1111/1471-0528.15796. Epub 2019 May 15. BJOG. 2019. PMID: 30989788 Clinical Trial.
-
Misoprostol vaginal insert versus misoprostol vaginal tablets for the induction of labour: a cohort study.BMC Pregnancy Childbirth. 2018 May 10;18(1):149. doi: 10.1186/s12884-018-1788-z. BMC Pregnancy Childbirth. 2018. PMID: 29747591 Free PMC article.
-
Misoprostol for induction of labor.Semin Perinatol. 2015 Oct;39(6):459-62. doi: 10.1053/j.semperi.2015.07.008. Semin Perinatol. 2015. PMID: 26601733 Review.
-
Balancing the efficacy and safety of misoprostol: a meta-analysis comparing 25 versus 50 micrograms of intravaginal misoprostol for the induction of labour.BJOG. 2015 Mar;122(4):468-76. doi: 10.1111/1471-0528.12935. Epub 2014 Jul 3. BJOG. 2015. PMID: 24989790 Review.
Cited by
-
The Efficacy of Misoprostol Vaginal Inserts for Induction of Labor in Women with Very Unfavorable Cervices.J Clin Med. 2023 Jun 17;12(12):4106. doi: 10.3390/jcm12124106. J Clin Med. 2023. PMID: 37373798 Free PMC article.
-
Comparison of Misoprostol for Labor Induction: Vaginal Insert Versus Oral Application Concerning Efficiency and Safety.In Vivo. 2024 Sep-Oct;38(5):2349-2357. doi: 10.21873/invivo.13701. In Vivo. 2024. PMID: 39187361 Free PMC article.
-
Results of Induction of Labor with Prostaglandins E1 and E2 (The RIPE Study): A Real-World Data Analysis of Obstetrical Effectiveness and Clinical Outcomes of Pharmacological Induction of Labor with Vaginal Inserts.Pharmaceuticals (Basel). 2023 Jul 8;16(7):982. doi: 10.3390/ph16070982. Pharmaceuticals (Basel). 2023. PMID: 37513894 Free PMC article.
-
Induction of Labor at Term with Oral Misoprostol or as a Vaginal Insert and Dinoprostone Vaginal Insert - A Multicenter Prospective Cohort Study.Geburtshilfe Frauenheilkd. 2022 Aug 10;82(8):868-873. doi: 10.1055/a-1860-0419. eCollection 2022 Aug. Geburtshilfe Frauenheilkd. 2022. PMID: 35967743 Free PMC article.
-
Induction of labour in nulliparous women- quick or slow: a cohort study comparing slow-release vaginal insert with low-dose misoprostol oral tablets.BMC Pregnancy Childbirth. 2020 Feb 7;20(1):79. doi: 10.1186/s12884-020-2770-0. BMC Pregnancy Childbirth. 2020. PMID: 32033600 Free PMC article.
References
-
- Tenore JL. Methods for cervical ripening and induction of labor. Am Fam Physician. 2003;67(10):2123–2128. - PubMed
-
- Mysodelle 200 micrograms vaginal delivery system SmPC. https://www.medicines.org.uk/emc/product/3436/smpc. Accessed 26 Oct 2018
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical