Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails
- PMID: 30374901
- PMCID: PMC6244569
- DOI: 10.1007/s40257-018-0384-3
Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails
Abstract
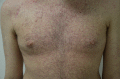
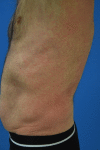
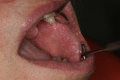
Targeted therapies and immunotherapies are associated with a wide range of dermatologic adverse events (dAEs) resulting from common signaling pathways involved in malignant behavior and normal homeostatic functions of the epidermis and dermis. Dermatologic toxicities include damage to the skin, oral mucosa, hair, and nails. Acneiform rash is the most common dAE, observed in 25-85% of patients treated by epidermal growth factor receptor and mitogen-activated protein kinase kinase inhibitors. BRAF inhibitors mostly induce secondary skin tumors, squamous cell carcinoma and keratoacanthomas, changes in pre-existing pigmented lesions, as well as hand-foot skin reactions and maculopapular hypersensitivity-like rash. Immune checkpoint inhibitors (ICIs) most frequently induce nonspecific maculopapular rash, but also eczema-like or psoriatic lesions, lichenoid dermatitis, xerosis, and pruritus. Of the oral mucosal toxicities observed with targeted therapies, oral mucositis is the most frequent with mammalian target of rapamycin (mTOR) inhibitors, followed by stomatitis associated to multikinase angiogenesis and HER inhibitors, geographic tongue, oral hyperkeratotic lesions, lichenoid reactions, and hyperpigmentation. ICIs typically induce oral lichenoid reactions and xerostomia. Targeted therapies and endocrine therapy also commonly induce alopecia, although this is still underreported with the latter. Finally, targeted therapies may damage nail folds, with paronychia and periungual pyogenic granuloma distinct from chemotherapy-induced lesions. Mild onycholysis, brittle nails, and a slower nail growth rate may also be observed. Targeted therapies and immunotherapies often profoundly diminish patients' quality of life, which impacts treatment outcomes. Close collaboration between dermatologists and oncologists is therefore essential.
Conflict of interest statement
Conflict of interest
Vincent Sibaud declares he received fees from Laboratoires dermatologiques Avène, Roche, Novartis, Bayer, Pierre Fabre and BMS. Mario Lacouture declares he has received consulting/advisory fees from Merck Sharp & Dohme Corporation, Galderma, Janssen Research & Development, LLC, Abbvie, Inc., Helsinn Healthcare SA, Novocure Inc., Boehringer Ingelheim Pharma GMBH & Co.KG, F. Hoffmann-La Roche AG, Allergan Inc., Amgen Inc., E.R. Squibb & Sons, L.L.C., Novartis Pharmaceuticals Corporation, EMD Serono, Inc., Astrazeneca Pharmaceuticals LP, Genentech, Inc, Leo Pharma Inc, Seattle Genetics, Debiopharm, Lindi, Bayer, Manner SAS, Menlo Ther, Celldex, Lutris, Pierre Fabre, Legacy Healthcare, Roche, Amryt Pharma, Johnson & Johnson, Paxman Coolers, Adjucare, Dignitana, Biotechspert, Parexel, Adgero, Veloce, Berg, US Biotest and research funding from Veloce, Berg, US Biotest, BMS.
Disclosure statement
This article is published as part of a journal supplement wholly funded by Laboratoires dermatologiques Avène.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

