Expression of odorant-binding proteins in mouthpart palps of the desert locust Schistocerca gregaria
- PMID: 30375079
- PMCID: PMC7380039
- DOI: 10.1111/imb.12548
Expression of odorant-binding proteins in mouthpart palps of the desert locust Schistocerca gregaria
Abstract
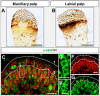
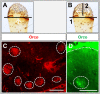
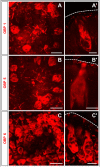
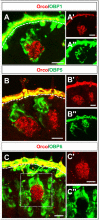
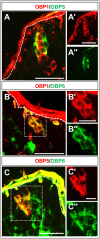
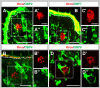
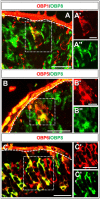
Odorant-binding proteins (OBPs) are essential molecular elements of the insect chemosensory system, which is composed of the antennae and the mouthpart palps (maxillary and labial). In this study, we have analysed the expression and the sensilla specificity of 14 OBP subtypes in the palps of the desert locust Schistocerca gregaria. The locust palps comprise only a low number of sensilla basiconica but a high number of sensilla chaetica. Employing a variety of approaches, we found that only a subset of the antennal OBP repertoire was expressed in both palp types. These OBPs were previously shown to be expressed either in sensilla basiconica or sensilla chaetica of the antennae. Comparing the expression pattern in the two chemosensory organs revealed similarities and differences; most remarkably, two OBP subtypes, OBP6 and OBP8, were found in both sensilla types on palps, whereas on the antennae they were solely expressed in one sensillum type. Together, the data indicate a differential, but partly overlapping, expression of OBPs in the two sensilla types of the palps. The differences in the expression pattern of OBP subtypes between antennae and palps might be indicative for distinct functions of the OBPs in the two chemosensory organs.
Keywords: desert locust; expression; insect; odorant-binding protein; palps.
© 2018 The Authors Insect Molecular Biology published by John Wiley & Sons Ltd on behalf of Royal Entomological Society.
Figures


Similar articles
-
The 40-Year Mystery of Insect Odorant-Binding Proteins.Biomolecules. 2021 Mar 30;11(4):509. doi: 10.3390/biom11040509. Biomolecules. 2021. PMID: 33808208 Free PMC article. Review.
-
SNMP1 and odorant receptors are co-expressed in olfactory neurons of the labial and maxillary palps from the desert locust Schistocerca gregaria (Orthoptera: Acrididae).Cell Tissue Res. 2020 Feb;379(2):275-289. doi: 10.1007/s00441-019-03083-x. Epub 2019 Aug 19. Cell Tissue Res. 2020. PMID: 31478139
-
Odorant Binding Proteins of the Desert Locust Schistocerca gregaria (Orthoptera, Acrididae): Topographic Expression Patterns in the Antennae.Front Physiol. 2018 Apr 17;9:417. doi: 10.3389/fphys.2018.00417. eCollection 2018. Front Physiol. 2018. PMID: 29719516 Free PMC article.
-
In Search for Pheromone Receptors: Certain Members of the Odorant Receptor Family in the Desert Locust Schistocerca gregaria (Orthoptera: Acrididae) Are Co-expressed with SNMP1.Int J Biol Sci. 2017 Jul 15;13(7):911-922. doi: 10.7150/ijbs.18402. eCollection 2017. Int J Biol Sci. 2017. PMID: 28808423 Free PMC article.
-
Elements of olfactory reception in adult Drosophila melanogaster.Anat Rec (Hoboken). 2013 Sep;296(9):1477-88. doi: 10.1002/ar.22747. Epub 2013 Jul 31. Anat Rec (Hoboken). 2013. PMID: 23904114 Review.
Cited by
-
Identification and comparative expression analysis of odorant-binding proteins in the reproductive system and antennae of Athetis dissimilis.Sci Rep. 2021 Jul 6;11(1):13941. doi: 10.1038/s41598-021-93423-1. Sci Rep. 2021. PMID: 34230568 Free PMC article.
-
Characterization of the ligand-binding properties of odorant-binding protein 38 from Riptortus pedestris when interacting with soybean volatiles.Front Physiol. 2025 Jan 6;15:1475489. doi: 10.3389/fphys.2024.1475489. eCollection 2024. Front Physiol. 2025. PMID: 39835200 Free PMC article.
-
The 40-Year Mystery of Insect Odorant-Binding Proteins.Biomolecules. 2021 Mar 30;11(4):509. doi: 10.3390/biom11040509. Biomolecules. 2021. PMID: 33808208 Free PMC article. Review.
-
Characterization of MaltOBP1, a Minus-C Odorant-Binding Protein, From the Japanese Pine Sawyer Beetle, Monochamus alternatus Hope (Coleoptera: Cerambycidae).Front Physiol. 2020 Apr 1;11:212. doi: 10.3389/fphys.2020.00212. eCollection 2020. Front Physiol. 2020. PMID: 32296339 Free PMC article.
-
Identification and analysis of odorant receptors expressed in the two main olfactory organs, antennae and palps, of Schistocerca americana.Sci Rep. 2022 Dec 31;12(1):22628. doi: 10.1038/s41598-022-27199-3. Sci Rep. 2022. PMID: 36587060 Free PMC article.
References
-
- Biessmann, H. , Nguyen, Q.K. , Le, D. and Walter, M.F. (2005) Microarray‐based survey of a subset of putative olfactory genes in the mosquito Anopheles gambiae . Insect Molecular Biology, 14, 575–589. - PubMed
-
- Blaney, W.M. (1974) Electrophysiological responses of the terminal sensilla on the maxillary palps of Locusta Migratoria (L.) to some electrolytes and non‐electrolytes. Journal of Experimental Biology, 60, 275–293. - PubMed
-
- Blaney, W.M. (1977) The ultrastructure of an olfactory sensillum on the maxillary palps of Locusta migratoria (L.). Cell and Tissue Research, 184, 397–409. - PubMed
-
- Blaney, W.M. and Chapman, R.F. (1969a) The fine structure of the terminal sensilla on the maxillary palps of Schistocerca gregaria (Forskål) (Orthoptera, Acrididae). Zeitschrift für Zellforsch und Mikroskopische Anat, 99, 74–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

