Interhemispheric connectivity during lateralized lexical decision
- PMID: 30375129
- PMCID: PMC6865399
- DOI: 10.1002/hbm.24414
Interhemispheric connectivity during lateralized lexical decision
Abstract
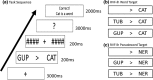
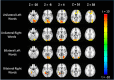
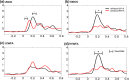
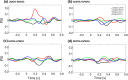
The well-established right visual field (RVF-lh) advantage in word recognition is commonly attributed to the typical left hemisphere dominance in language; words presented to the LVF-rh are processed less efficiently due to the need for transcallosal transfer from the right to left hemisphere. The exact stage for this hemispheric transfer is currently unsettled. Some studies suggest that transfer occurs at very early stages between primary visual regions, whereas other studies suggest that transfer occurs between the left visual word form area and its right hemisphere homolog. This study explores these conflicting accounts and finds evidence for both. Participants conducted a lateralized lexical decision task with both unilateral and bilateral display conditions. Connectivity analyses were conducted from magnetoencephalography signals that were localized to the left middle occipital gyrus (LMOG), right middle occipital gyrus (RMOG), left visual word form area (LVWFA), and right visual word form area (RVWA). Results from unilateral trials showed asymmetrical interhemispheric connectivity from the RMOG to LMOG and symmetrical interhemispheric connectivity between the LVWFA and RVWFA. Furthermore, bilateral presentations led to reduced interhemispheric connectivity between both homologous region of interest pairs. Together, these results suggest that lateralized word recognition involves multiple stages of interhemispheric interactions and that these interactions are reduced with bilateral displays.
Keywords: functional connectivity; hemispheric interactions; visual word recognition.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures






References
-
- Banich, M. T. (2003). The Divided Visual Field Technique in Laterality and Interhemispheric Integration In Hugdahl K. (Ed.), Experimental Methods in Neuropsychology (pp. 47–63). Springer US; 10.1007/978-1-4615-1163-2_3 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

