Evidence of natural Zika virus infection in neotropical non-human primates in Brazil
- PMID: 30375482
- PMCID: PMC6207778
- DOI: 10.1038/s41598-018-34423-6
Evidence of natural Zika virus infection in neotropical non-human primates in Brazil
Abstract
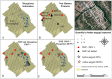
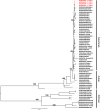
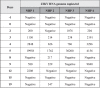
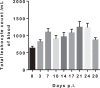
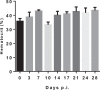
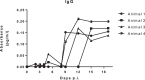
In Africa, Old World Primates are involved in the maintenance of sylvatic circulation of ZIKV. However, in Brazil, the hosts for the sylvatic cycle remain unknown. We hypothesized that free-living NHPs might play a role in urban/periurban ZIKV dynamics, thus we undertook an NHP ZIKV investigation in two cities in Brazil. We identified ZIKV-positive NHPs and sequences obtained were phylogenetically related to the American lineage of ZIKV. Additionally, we inoculated four C. penicillata with ZIKV and our results demonstrated that marmosets had a sustained viremia. The natural and experimental infection of NHPs with ZIKV, support the hypothesis that NHPs may be a vertebrate host in the maintainance of ZIKV transmission/circulation in urban tropical settings. Further studies are needed to understand the role they may play in maintaining the urban cycle of the ZIKV and how they may be a conduit in establishing an enzootic transmission cycle in tropical Latin America.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

