PINK1-dependent mitophagy is driven by the UPS and can occur independently of LC3 conversion
- PMID: 30375512
- PMCID: PMC6748138
- DOI: 10.1038/s41418-018-0219-z
PINK1-dependent mitophagy is driven by the UPS and can occur independently of LC3 conversion
Abstract
The Parkinson's disease (PD)-related ubiquitin ligase Parkin and mitochondrial kinase PINK1 function together in the clearance of damaged mitochondria. Upon mitochondrial depolarization, Parkin translocates to mitochondria in a PINK1-dependent manner to ubiquitinate outer mitochondrial membrane proteins. According to the current model, the ubiquitin- and LC3-binding adaptor protein SQSTM1 is recruited to mitochondria, followed by their selective degradation through autophagy (mitophagy). However, the role of the ubiquitin proteasome system (UPS), although essential for this process, still remains largely elusive. Here, we investigated the role of the UPS and autophagy by applying the potassium ionophore Valinomycin in PINK1-deficient human fibroblasts and isogenic neuroblastoma cell lines generated by CRISPR/Cas9. Although identical to the commonly used CCCP/FCCP in terms of dissipating the mitochondrial membrane potential and triggering complete removal of mitochondria, Valinomycin did not induce conversion of LC3 to its autophagy-related form. Moreover, FCCP-induced conversion of LC3 occurred even in mitophagy-incompetent, PINK1-deficient cell lines. While both stressors required a functional UPS, the removal of depolarized mitochondria persisted in cells depleted of LC3A and LC3B. Our study highlights the importance of the UPS in PINK1-/Parkin-mediated mitochondrial quality control. In contrast, activation of autophagy, monitored through conversion of LC3, is likely induced by depolarizing-agent-induced toxicity in a PINK1-/Parkin-independent manner.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

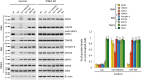






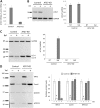

Similar articles
-
PINK1 is recruited to mitochondria with parkin and associates with LC3 in mitophagy.FEBS Lett. 2010 Mar 19;584(6):1073-9. doi: 10.1016/j.febslet.2010.02.016. Epub 2010 Feb 12. FEBS Lett. 2010. PMID: 20153330
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
-
Phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)-dependent ubiquitination of endogenous Parkin attenuates mitophagy: study in human primary fibroblasts and induced pluripotent stem cell-derived neurons.J Biol Chem. 2013 Jan 25;288(4):2223-37. doi: 10.1074/jbc.M112.391680. Epub 2012 Dec 4. J Biol Chem. 2013. PMID: 23212910 Free PMC article.
-
Constitutive Activation of PINK1 Protein Leads to Proteasome-mediated and Non-apoptotic Cell Death Independently of Mitochondrial Autophagy.J Biol Chem. 2016 Jul 29;291(31):16162-74. doi: 10.1074/jbc.M116.714923. Epub 2016 Jun 14. J Biol Chem. 2016. PMID: 27302064 Free PMC article.
-
The ubiquitin signal and autophagy: an orchestrated dance leading to mitochondrial degradation.EMBO Rep. 2016 Mar;17(3):300-16. doi: 10.15252/embr.201541486. Epub 2016 Feb 8. EMBO Rep. 2016. PMID: 26882551 Free PMC article. Review.
Cited by
-
Therapeutic Potential of Exploiting Autophagy Cascade Against Coronavirus Infection.Front Microbiol. 2021 May 14;12:675419. doi: 10.3389/fmicb.2021.675419. eCollection 2021. Front Microbiol. 2021. PMID: 34054782 Free PMC article. Review.
-
UBQLN2 and HSP70 participate in Parkin-mediated mitophagy by facilitating outer mitochondrial membrane rupture.EMBO Rep. 2023 Sep 6;24(9):e55859. doi: 10.15252/embr.202255859. Epub 2023 Jul 28. EMBO Rep. 2023. PMID: 37501540 Free PMC article.
-
HIF-1 signaling: an emerging mechanism for mitochondrial dynamics.J Physiol Biochem. 2023 Aug;79(3):489-500. doi: 10.1007/s13105-023-00966-0. Epub 2023 May 13. J Physiol Biochem. 2023. PMID: 37178248 Review.
-
Mitochondrial dynamics during spermatogenesis.J Cell Sci. 2020 Jul 16;133(14):jcs235937. doi: 10.1242/jcs.235937. J Cell Sci. 2020. PMID: 32675215 Free PMC article. Review.
-
Synergistic effects of autophagy/mitophagy inhibitors and magnolol promote apoptosis and antitumor efficacy.Acta Pharm Sin B. 2021 Dec;11(12):3966-3982. doi: 10.1016/j.apsb.2021.06.007. Epub 2021 Jun 16. Acta Pharm Sin B. 2021. PMID: 35024319 Free PMC article.
References
-
- Rakovic A, et al. Phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)-dependent ubiquitination of endogenous Parkin attenuates mitophagy: study in human primary fibroblasts and induced pluripotent stem cell-derived neurons. J Biol Chem. 2013;288:2223–37. doi: 10.1074/jbc.M112.391680. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

