Exocytosis of Weibel-Palade bodies: how to unpack a vascular emergency kit
- PMID: 30375718
- PMCID: PMC7379738
- DOI: 10.1111/jth.14322
Exocytosis of Weibel-Palade bodies: how to unpack a vascular emergency kit
Abstract
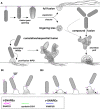
The blood vessel wall has a number of self-healing properties, enabling it to minimize blood loss and prevent or overcome infections in the event of vascular trauma. Endothelial cells prepackage a cocktail of hemostatic, inflammatory and angiogenic mediators in their unique secretory organelles, the Weibel-Palade bodies (WPBs), which can be immediately released on demand. Secretion of their contents into the vascular lumen through a process called exocytosis enables the endothelium to actively participate in the arrest of bleeding and to slow down and direct leukocytes to areas of inflammation. Owing to their remarkable elongated morphology and their secretory contents, which span the entire size spectrum of small chemokines all the way up to ultralarge von Willebrand factor multimers, WPBs constitute an ideal model system for studying the molecular mechanisms of secretory organelle biogenesis, exocytosis, and content expulsion. Recent studies have now shown that, during exocytosis, WPBs can undergo several distinct modes of fusion, and can utilize fundamentally different mechanisms to expel their contents. In this article, we discuss recent advances in our understanding of the composition of the WPB exocytotic machinery and how, because of its configuration, it is able to support WPB release in its various forms.
Keywords: Weibel-Palade bodies; endothelial cells; exocytosis; von Willebrand disease; von Willebrand factor.
© 2018 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals, Inc. on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

