Protocol Paper: Oral Poliovirus Vaccine Transmissibility in Communities After Cessation of Routine Oral Poliovirus Vaccine Immunization
- PMID: 30376084
- PMCID: PMC6206104
- DOI: 10.1093/cid/ciy606
Protocol Paper: Oral Poliovirus Vaccine Transmissibility in Communities After Cessation of Routine Oral Poliovirus Vaccine Immunization
Abstract
Background: We aimed to elucidate household and community-level shedding and transmission of trivalent oral polio vaccine (tOPV) in communities with inactivated polio vaccine (IPV) routine immunization after tOPV is administered during a national health week (NHW).
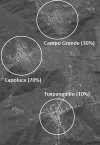
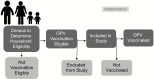
Methods: We conducted a 3-arm, randomized trial with data collected at baseline through 10 weeks post-NHW in households with at least 1 child <5 years old in 3 semi-rural communities in Orizaba, Mexico. Selected communities were geographically isolated but socio-demographically similar. Each community was assigned an oral polio vaccine (OPV) immunization rate: 10, 30, or 70% of participating households. From 2653 households in the 3 communities, ~150 households per community were selected, for 466 in total. Households were randomized as vaccinated or unvaccinated, with only 1 child under 5 in the vaccinated household receiving OPV during the February 2015 NHW. No other community members received OPV during this NHW. Stool samples were collected up to 10 weeks post-vaccination for all members of the 466 study households and were analyzed for the presence of OPV serotypes using a multiplex polymerase chain reaction assay.
Results: We will report on the factors associated with, and incidence and duration of, household and community shedding and transmission of OPV. The secondary outcomes will characterize temporal and geospatial OPV serotype shedding patterns.
Conclusions: The current global polio eradication plan relies on transitioning away from OPV to IPV. This study contributes to understanding patterns of OPV shedding and transmission dynamics in communities with primary IPV immunity, in order to optimize the reduction of OPV transmission.
Figures
Similar articles
-
Characterization of Household and Community Shedding and Transmission of Oral Polio Vaccine in Mexican Communities With Varying Vaccination Coverage.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S4-S17. doi: 10.1093/cid/ciy650. Clin Infect Dis. 2018. PMID: 30376097 Free PMC article. Clinical Trial.
-
Spatial Analyses of Oral Polio Vaccine Transmission in an Community Vaccinated With Inactivated Polio Vaccine.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S18-S25. doi: 10.1093/cid/ciy622. Clin Infect Dis. 2018. PMID: 30376089 Free PMC article. Clinical Trial.
-
Assessing the individual risk of fecal poliovirus shedding among vaccinated and non-vaccinated subjects following national health weeks in Mexico.PLoS One. 2017 Oct 12;12(10):e0185594. doi: 10.1371/journal.pone.0185594. eCollection 2017. PLoS One. 2017. PMID: 29023555 Free PMC article. Clinical Trial.
-
Immunogenicity of New Primary Immunization Schedules With Inactivated Poliovirus Vaccine and Bivalent Oral Polio Vaccine for the Polio Endgame: A Review.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S35-S41. doi: 10.1093/cid/ciy633. Clin Infect Dis. 2018. PMID: 30376081 Free PMC article. Review.
-
Vaccine schedules and the effect on humoral and intestinal immunity against poliovirus: a systematic review and network meta-analysis.Lancet Infect Dis. 2019 Oct;19(10):1121-1128. doi: 10.1016/S1473-3099(19)30301-9. Epub 2019 Jul 23. Lancet Infect Dis. 2019. PMID: 31350192
Cited by
-
Lab Protocol Paper: Use of a High-throughput, Multiplex Reverse-transcription Quantitative Polymerase Chain Reaction Assay for Detection of Sabin Oral Polio Vaccine in Fecal Samples.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S121-S126. doi: 10.1093/cid/ciy648. Clin Infect Dis. 2018. PMID: 30376092 Free PMC article.
-
Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization.J Control Release. 2019 Jun 10;303:130-150. doi: 10.1016/j.jconrel.2019.04.025. Epub 2019 May 3. J Control Release. 2019. PMID: 31022431 Free PMC article. Review.
-
Rapid emergence and transmission of virulence-associated mutations in the oral poliovirus vaccine following vaccination campaigns.NPJ Vaccines. 2023 Sep 25;8(1):137. doi: 10.1038/s41541-023-00740-9. NPJ Vaccines. 2023. PMID: 37749086 Free PMC article.
-
Rapid emergence and transmission of virulence-associated mutations in the oral poliovirus vaccine following vaccination campaigns.medRxiv [Preprint]. 2023 Mar 21:2023.03.16.23287381. doi: 10.1101/2023.03.16.23287381. medRxiv. 2023. Update in: NPJ Vaccines. 2023 Sep 25;8(1):137. doi: 10.1038/s41541-023-00740-9. PMID: 36993386 Free PMC article. Updated. Preprint.
-
Assessing the Risk of Vaccine-derived Outbreaks After Reintroduction of Oral Poliovirus Vaccine in Postcessation Settings.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S26-S34. doi: 10.1093/cid/ciy605. Clin Infect Dis. 2018. PMID: 30376087 Free PMC article.
References
-
- GPEI. The polio eradication and endgame strategic plan 2013–2018 Available at: http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf. Accessed December 2016.
-
- Organization WH. Polio global eradication initiative annual report 2015 Available at: http://polioeradication.org/wp-content/uploads/2016/10/AR2015.pdf. Accessed 4 May 2016.
-
- Orelien J. Model Fitting in PROC GENMOD. SAS Users Group Internation. Statistics, Data Analysis, and Data Mining 264-26, 2001. Available at: http://www2.sas.com/proceedings/sugi26/p264-26.pdf
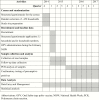
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical