Assessing the Risk of Vaccine-derived Outbreaks After Reintroduction of Oral Poliovirus Vaccine in Postcessation Settings
- PMID: 30376087
- PMCID: PMC6206116
- DOI: 10.1093/cid/ciy605
Assessing the Risk of Vaccine-derived Outbreaks After Reintroduction of Oral Poliovirus Vaccine in Postcessation Settings
Abstract
Background: The Polio Eradication and Endgame Strategic Plan 2013-2018 calls for the gradual withdrawal of oral poliovirus vaccine (OPV) from routine immunization. We aimed to quantify the transmission potential of Sabin strains from OPV when it is reintroduced, accidentally or deliberately, in a community vaccinated with inactivated poliovirus vaccine alone.
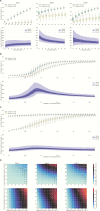
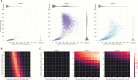
Methods: We built an individual-based stochastic epidemiological model that allows independent spread of 3 Sabin serotypes and differential transmission rates within versus between households. Model parameters were estimated by fitting to data from a prospective cohort in Mexico. We calculated the effective reproductive number for the Mexico cohort and simulated scenarios of Sabin strain resurgence under postcessation conditions, projecting the risk of prolonged circulation, which could lead to circulating vaccine-derived poliovirus (cVDPV).
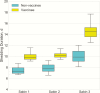
Results: The estimated effective reproductive number for naturally infected individuals was about 1 for Sabin 2 and Sabin 3 (OPV2 and OPV3) in a postcessation setting. Most transmission events occurred between households. We estimated the probability of circulation for >9 months to be (1) <<1% for all 3 serotypes when 90% of children <5 years of age were vaccinated in a hypothetical outbreak control campaign; (2) 45% and 24% for Sabin 2 and Sabin 3, respectively, when vaccine coverage dropped to 10%; (3) 37% and 8% for Sabin 2 and Sabin 3, respectively, when a single active shedder appeared in a community.
Conclusions: Critical factors determining the risk of cVDPV emergence are the scale at which OPV is reintroduced and the between-household transmission rate for poliovirus, with intermediate values posing the greatest risk.
Figures

Similar articles
-
Assessing the stability of polio eradication after the withdrawal of oral polio vaccine.PLoS Biol. 2018 Apr 27;16(4):e2002468. doi: 10.1371/journal.pbio.2002468. eCollection 2018 Apr. PLoS Biol. 2018. PMID: 29702638 Free PMC article.
-
[Circulating vaccine-derived poliovirus type 2 outbreak in Democratic Republic of Congo 2011-2012].Bull Soc Pathol Exot. 2015 Oct;108(4):235-41. doi: 10.1007/s13149-015-0447-4. Epub 2015 Aug 18. Bull Soc Pathol Exot. 2015. PMID: 26288132 French.
-
Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of inadvertent trivalent oral poliovirus vaccine use.BMC Infect Dis. 2016 Jun 1;16:237. doi: 10.1186/s12879-016-1537-8. BMC Infect Dis. 2016. PMID: 27246198 Free PMC article.
-
Updated Characterization of Post-OPV Cessation Risks: Lessons from 2019 Serotype 2 Outbreaks and Implications for the Probability of OPV Restart.Risk Anal. 2021 Feb;41(2):320-328. doi: 10.1111/risa.13555. Epub 2020 Jul 6. Risk Anal. 2021. PMID: 32632925 Free PMC article. Review.
-
Responding to a cVDPV1 outbreak in Ukraine: Implications, challenges and opportunities.Vaccine. 2017 Aug 24;35(36):4769-4776. doi: 10.1016/j.vaccine.2017.04.036. Epub 2017 May 19. Vaccine. 2017. PMID: 28528761 Free PMC article. Review.
Cited by
-
The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study.Lancet. 2019 Jul 13;394(10193):148-158. doi: 10.1016/S0140-6736(19)31279-6. Epub 2019 Jun 4. Lancet. 2019. PMID: 31174831 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of inactivated poliovirus vaccine schedules for the post-eradication era: a randomised open-label, multicentre, phase 3, non-inferiority trial.Lancet Infect Dis. 2021 Apr;21(4):559-568. doi: 10.1016/S1473-3099(20)30555-7. Epub 2020 Oct 23. Lancet Infect Dis. 2021. PMID: 33284114 Free PMC article. Clinical Trial.
-
Is it time to switch to a formulation other than the live attenuated poliovirus vaccine to prevent poliomyelitis?Front Public Health. 2024 Jan 8;11:1284337. doi: 10.3389/fpubh.2023.1284337. eCollection 2023. Front Public Health. 2024. PMID: 38259741 Free PMC article. Review.
-
Review of poliovirus modeling performed from 2000 to 2019 to support global polio eradication.Expert Rev Vaccines. 2020 Jul;19(7):661-686. doi: 10.1080/14760584.2020.1791093. Epub 2020 Aug 1. Expert Rev Vaccines. 2020. PMID: 32741232 Free PMC article. Review.
-
Economic assessment of incorporating the hexavalent vaccine as part of the National Immunization Program of Peru.BMC Health Serv Res. 2022 May 16;22(1):651. doi: 10.1186/s12913-022-08006-1. BMC Health Serv Res. 2022. PMID: 35570278 Free PMC article.
References
-
- World Health Organization. Global polio eradication initiative annual report 2017. 2017. Available at: http://polioeradication.org/wp-content/uploads/2018/09/polio-eradication.... Accessed 25 September 2018.
-
- Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine—Worldwide, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:934–8. - PubMed
-
- Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-derived polioviruses. J Infect Dis 2014; 210(suppl 1):S283–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

